Summary
Recent data from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial indicate a bell-shaped curve association between an increase in endomyocardial injections and the therapeutic effect of cardiopoetic, mesenchymal stem-cell transplantation in patients with chronic ischemic heart failure. In our study with a median of 20 injections per patient, a decrease in regional contractility was observed in nearly one-third of the segments analyzed; this was only partially reversible by 30 days. These findings may provide a mechanistic explanation for the reduction of the therapeutic effect of cardiopoetic cells with an increased number of cell-delivery trans-endocardial injections (bell-shaped curve).
Introduction
The long-term prognosis of chronic ischemic heart failure (iCHF) remains poor despite advances in diagnosis and treatment [1]. Stem cell-based therapies are investigated as a tool to stimulate myocardial regeneration in iCHF and potentially reverse myocardial damage [2–5]. Despite promising preclinical data, stem cell-based therapy trials have shown only a modest improvement in left ventricular ejection fraction (LVEF) and left ventricular remodeling in patients with iCHF [2, 6]. Clinical trials have often used the transendocardial route (transendocardial injections) [7–9] to deliver therapeutic agents to the myocardium in iCHF [10–12].
Recent data from the CHART-1 study indicates that the therapeutic benefit of transendocardial cell transplantation may be lost with an increasing number of injections [3], suggesting that endomyocardial administration of stem cells may elicit a degree of mechanical myocardial damage – a “multiplied” effect of myocardial injury elicited with myocardial biopsy [13, 14].
Aim
The study aimed to evaluate regional and global left ventricular contractility and diastolic function in patients with advanced symptomatic ischemic heart failure who underwent trans-endomyocardial administration of cardiopoetic stem cells (CSCs) and compared to a sham procedure. Echocardiographic outcomes of both procedures were evaluated on days 1 and 30 after the index procedure.
Material and methods
Study population
We investigated ten consecutive patients (mean age: 60.8 ±7.1 years) with chronic heart failure of ischemic etiology not eligible for revascularization, with left ventricular ejection fraction (LVEF) below 35% by echocardiography and a history of hospitalization for heart failure within 12 months. Consecutive patients meeting inclusion criteria were recruited at the Department of Cardiac and Vascular Diseases of the John Paul II Hospital, Krakow, Poland. Exclusion criteria were recent myocardial infarction or unstable angina, recent percutaneous or surgical coronary revascularization, cardiac resynchronization therapy between 3 and 6 months prior to enrollment, and New York Heart Association (NYHA) class IV [2, 3]. All patients gave informed consent before study enrollment. The local Ethics Committee approved the study protocol. Patients were externally randomized to endomyocardial delivery of cardiopoietic cells or a sham procedure; the present study was an investigator-initiated substudy of the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) Trial [2].
Intervention
Mesenchymal stem cells were harvested from bone marrow and cultured in a central laboratory to achieve at least 24 million autologous CSCs from a harvest. Transendocardial injections (up to 20; 0.5 ml each) were administered into left ventricular wall segments with impaired contractility and a wall thickness ≥ 8 mm, avoiding the area of the mitral and aortic valves. Target zones were chosen and confirmed with left ventricular angiography and periprocedural echocardiography. The procedure was done with C-Cath, curved, multi-perforated needle (side holes) catheter, designed to enhance cell delivery [15, 16]. For a sham procedure, a pigtail catheter was placed in the left ventricular cavity, and saline injections were performed ‘targeting’ specific zones as in active procedures (Figures 1, 2). Patients were followed for 30 days after the procedure for procedure-related adverse events.
Figure 1
An example of an intracardiac, trans-catheter, needle-based, cardiopoetic stem-cell delivery procedure. A, B – Ventriculography (A – antero-posterior (AP) and B – left anterior oblique (LAO) view) prior to trans-endocardial cell delivery; black arrows indicate the tip of pigtail catheter; C, D – injection catheter attached to the target cardiac wall in the corresponding projection (C – AP, D – LAO view); white arrows indicate the tip of a needle-tipped catheter for cell delivery
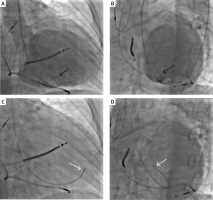
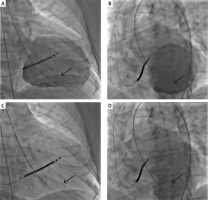
Figure 2
Example of a ‘sham procedure’. A, B – Ventriculography (A – antero-posterior (AP) and B – left anterior oblique (LAO) view); C, D – Sham procedure, pigtail catheter positioned in the corresponding projections (C – AP, D – LAO view); low volume saline injections (n = 15-20) mimicking CSCs delivery
Myocardial imaging
Left ventricular contractility, systolic and diastolic volumes, and parameters of diastolic function (peak early (E) and late (A) diastolic transmitral velocity, E deceleration time, E/A ratio, peak early annular myocardial diastolic velocity (E′), and E/E′ ratio) were acquired with two-dimensional , pulse-wave Doppler, and tissue Doppler imaging echocardiography (Vivid 7 Digital Ultrasound System, GE, Healthcare, Little Chalfont, UK). Wall motion was semiquantitatively evaluated using a 17-segment model based on three long-axis views and scored 1 if normal, 2 for hypokinetic, 3 for severely hypokinetic or akinetic, and 4 points for a dyskinetic segment. The wall motion score index (WMSI) was estimated as the ratio of the sum of the wall motion scores divided by the number of myocardial segments scored [17].
All measurements were obtained according to the guidelines of the European Association of Cardiovascular Imaging [17, 18] at baseline, after index procedure and at 30-day follow-up. Two experts blinded to the procedure type analyzed the measurements according to consensus.
Statistical analysis
Categorical variables were expressed as numbers and percentages and compared using the χ2 test and Fisher’s exact test. Continuous variables were presented as a mean ± standard deviation (SD) or a median and interquartile range (IQR). Continuous data were assessed for normality of distribution using the Shapiro-Wilk test and compared using Student’s t-test or Mann-Whitney test, as appropriate. Within-group continuous data were evaluated with one-factor repeated measures ANOVA and Tukey’s HSD test or Friedman’s test, as appropriate. Relations between two continuous variables were evaluated using Pearson’s or Spearman’s correlations for normally or non-normally distributed variables, respectively. Probability values < 0.05 were considered statistically significant. All data were evaluated using Statistica 13.3 software (StatSoft Inc, Tulsa, OK).
Results
Characteristics of patients
The cohort of patients with iCHF treated with CSCs or placebo (sham procedure) is presented in Table I. Patients in the CSCs group had a longer procedure time and a higher radiation dose compared to the sham procedure group. Both groups were well balanced regarding demographic data, comorbidities, treatment, and echocardiographic variables (Tables I, II).
Table I
Baseline characteristics of patients
[i] Values are presented as n (%), mean ± standard deviation, or median (interquartile range). ACEI – angiotensin-converting enzyme inhibitor, ARB – angiotensin II receptor antagonist, BMI – body mass index, CABG – coronary artery bypass grafting, CSCs – cardiopoetic stem cells, ICD – implantable cardioverter-defibrillator, IRA – infarct-related artery, LAD/Cx/RCA – left anterior descending/circumflex/right coronary artery, MRA – mineralocorticoid receptor antagonist, TIA – transient ischemic attack.
Table II
Echocardiographic data during follow-up
| Parameter | CSCs group (n = 5) | Sham procedure group (n = 5) | CSCs vs. sham procedure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Day 1 | Day 30 | P-value | Baseline | Day 1 | Day 30 | P-value | Baseline p-value | P-value Day 1 | P-value Day 30 | |
| LVEF (%) | 22.8 ±4.5 | 19.6 ±4.5 | 20.5 ±5.0 | 0.067 | 27.8 ±5.8 | 25.4 ±8.3 | 24.9 ±9.0 | 0.346 | 0.501 | 0.362 | 0.307 |
| WMSI | 2.26 ±0.16 | 2.58 ±0.12* | 2.46 ±0.19* | 0.003 | 2.29 ±0.26 | 2.35 ±0.24 | 2.33 ±0.28 | 0.450 | 0.859 | 0.099 | 0.413 |
| LVEDV [ml] | 198.0 (193.8–205.6) | 208.9 (184.0–213.1) | 211.0 (176.6–212.8) | 0.246 | 201.1 (189.7–226.4) | 189.0 (185.4–216.1) | 193.0 (185.8–226.1) | 0.067 | 0.624 | 0.230 | 0.749 |
| LVESV [ml] | 136.3 (135.6–153.2) | 136.7 (130.3–153.7) | 137.4 (132.9–156.1) | 0.165 | 162.5 (140.9–164.5) | 176.6 (139.5–180.1) | 172.9 (130.6–173.1) | 0.548 | 0.928 | 0.667 | 0.841 |
| Transmitral E velocity [cm/s] | 67.6 ±22.3 | 84.6 ±46.7 | 72.0 ±38.2 | 0.253 | 73.2 ±24.4 | 63.2 ±25.5 | 73.2 ±19.8 | 0.747 | 0.715 | 0.394 | 0.965 |
| Transmitral E deceleration time [ms] | 212.6 ±97.4 | 199.8 ±63.4 | 174.6 ±51.5 | 0.504 | 222.6 ±47.4 | 246 ±56.8 | 190.2 ±72.9 | 0.280 | 0.841 | 0.260 | 0.707 |
| Transmitral E/A ratio | 1.1 (0.9–2.8) | 1.6 (0.9–4.2) | 1.5 (0.5–3.5) | 0.449 | 1.3 (0.9–1.8) | 0.8 (0.5–1.1) | 0.7 (0.5–1.0) | 0.472 | 0.750 | 0.334 | 0.213 |
| Mean ratio of E/e′ | 17.6 ±12.3 | 17.1 ±11.7 | 14.0 ±9.5 | 0.154 | 19.1 ±6.1 | 17.5 ±9.4 | 22.5 ±10.4 | 0.444 | 0.810 | 0.946 | 0.215 |
* Post hoc test p-value vs. baseline < 0.05. For abbreviations, see Table I;
CSCs delivery to the targeted segments
A total of 92 injections of 0.5 ml of CSCs were performed in 48 of 85 pre-mapped segments (56.5%).
The target zones were located in the lateral n = 13 (27.1%), inferior n = 10 (20.8%), anterior n = 10 (16.7%), anteroseptal n = 8 (16.7%), inferolateral n = 4 (8.3%) and inferoseptal wall n = 3 (6.25%) of the left ventricle. Patients No. 1–5 had 5, 12, 12, 10, and 9 targeted segments with 15, 20, 20, 20, and 17 injections, respectively.
Regional left ventricular systolic function
A total of 170 segments were analyzed. WMSI on day 1 and day 30 increased by 0.32 ±0.06 and 0.19 ±0.18 (p = 0.02, p = 0.03, respectively) compared to baseline in the CSCs group and did not change in the sham group (0.06 ±0.06, p = 0.42, for day 1; 0.04 ±0.11, p = 0.72, for day 30) (Table II, Figure 3). With a similar number of per patient injections in this substudy a decrease in regional contractility (wall motion score reduction ≥ 1) was observed in the CSCs group in 26/85 (30.6%) on days 0 and in 16/85 (18.9%) on day 30 of pre-mapped segments, including 19/48 (39.6%) and 11/48 (22.9%) targeted segments and 7/37 (18.9%) and 5/37 (13.5%) non-targeted segments, respectively. Deterioration was observed most frequently in the anteroseptal (n = 7, 46.7%), then anterior, lateral, and inferior wall segments (all n = 5, 33.3%). The magnitude of deterioration was reduced at 30 days (Figure 3 A).
Figure 3
30-day evolution of left ventricular systolic function after transendocardial stem cells delivery (perforated needle injections) (A, C) and in the sham procedure group (B, D)
WMSI – the wall motion score index, LVEF – left ventricular ejection fraction.
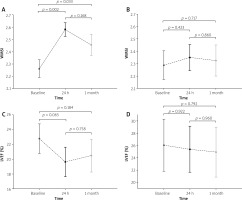
In the sham group, a decrease in contractility was observed in 11.7% (n = 10/85, day 0) and 10.6% of the segments (n = 9/85, day 30), while an increase in contractility was observed in 5.9% (n = 5/85, for days 0 and 30).
Left ventricular ejection fraction and diastolic function
There was a reduction of LVEF in the CSCs group on day 1 (–3.15 ±1.23%, p = 0.065) and on day 30 (–2.29 ±2.51%, p = 0.18) that, however, did not reach statistical significance with n = 5. LVEF did not change significantly in the sham group (–0.66 ±3.01%, p = 0.92; –1.14 ±2.84%, p = 0.79, respectively) (Figure 3). A weak, non-significant negative correlation was observed between the number of injections and LVEF on days 1 and 30 (r = –0.78, p = 0.12 for both). We did not observe differences in transmitral E velocity, E wave deceleration time, E/A ratio, and E/e′ mean ratio on days 1 and 30 (Table II).
No serious or non-serious adverse clinical events occured during the 30-day follow-up.
Discussion
This prospective study shows that trans-endomyocardial administration of CSCs in patients with iCHF was associated with a systolic function impairment in the targeted segments. This phenomenon was particularly relevant in the anteroseptal wall and resolved in a large part, though not complately, during the 30-day follow-up.
No prolonged ischemia, including prolonged chest pain, or ischemic changes on the electrocardiogram occurred. Furthermore, we did not observe a worsening of diastolic function that precedes systolic dysfunction during ischemia [19]. The duration and dose of radiation of the procedure, (an index of procedural complexity)might have played a contributory role [20].
In a recent study, neurogenic contractility impairment was observed in nearly 25% of patients with acute ischaemic myocardial damage [21]. This effect is unlikely to play any important role in our present study that was sham-procedure controlled. Furthermore it needs to be taken into consideration that the precision of CSCs delivery to target zones may be limited as angiography or standard transthoracic echocardiograpy precision cannot provide real-time three-dimensional images.
We assume that CSCs, despite their regenerative potential, immunoregulatory properties, and ability to improve myocardial perfusion and left ventricular function [22], may not fully compensate periprocedural damage caused by endomyocardial needle-based delivery, as we observed a residual increase in WMSI on day 30 after the procedure. This may be partially related to the increased number of injections. The effect we have identified may be applicable to other trials such as, most recently, the SCIENCE study [23] that unfortunately did not provide troponin levels in relation to trans-endocardial cell delivery.
In summary, our findings suggest that endomyocardial administration of CSCs in iCHF in humans might be associated with prolonged deterioration of regional systolic function. In the targeted segments, procedure-related injury largely, but not completely resolved by 30 days.
This study has several limitations. First, the size of our substudy was limited. Second, the protocol did not include multimodal imaging to evaluate left ventricular function; however, our recent study showed a strong concordance between LVEF derived from echocardiography, single-photon emission computed tomography, and cardiac magnetic resonance imaging [24]. Third, cardiac biomarkers although sampled were not available to the present analysis. Fourth, the statistical associations described here do not necessarily indicate cause-and-effect relationships. Finally, elucidation of the mechanisms underlying systolic dysfunction after endomyocardial administration of CSCs was beyond the scope of this study and the sample size does not permit analysis by the number of injections.
Conclusions
Our findings demonstrate that in patients with iCHF, endomyocardial delivery of mesenchymal stem cells is associated with transient regional left ventricular systolic dysfunction observed the day after the procedure and its gradual, though not complete, resolution within 30 days. Taken together with other limitations of the transendocardial “needle-based” approach (including the ‘geographic’ limitations related to the anatomy of the endocardial surface of the myocardium and the very focal nature of therapeutic agent delivery), transcoronary delivery techniques might be preferable once the terapeutic cells uptake is confirmed with labeling techniques [25].








