Transcatheter mitral valve-in-valve intervention (TMVI) is an alternative mode of treatment to re-do surgery for bioprosthetic valve failure. Although transapical access represents the shortest route to deliver the new bioprosthesis in the mitral position, currently the transseptal route is becoming more popular [1, 2]. We present the first case in Poland of TMVI using transseptal access.
A 69-year-old female patient who underwent surgical mitral valve replacement with an Epic 31 mm (Abbot) valve, after 2 ischemic strokes with pulmonary hypertension and chronic kidney disease stage 4 (EuroSCORE II 9.8%), developed dyspnea 3 years following surgery (NYHA class III). Echocardiographic examination revealed signs of severe stenosis and mild insufficiency of the Epic valve. After discussion at the heart team meeting, due to high risk of a re-do operation the patient was referred for TMVI.
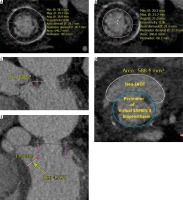
Based on the internal lumen diameter of the Epic valve the suggested valve size should be 29 mm (Figure 1 A), but cardiac computed tomography (CCT) revealed extensive hypodense pannus formation with mean inner lumen diameter of 21 mm (Figure 1 B). Because of this appearance it was agreed to perform balloon sizing of the degenerated bioprosthesis with a 22 mm balloon catheter. The patient was screened for possible left ventricular outflow tract (LVOT) obstruction by means of neo-LVOT calculation and aorto-mitral angulation, showing no or little risk of obstruction (Figures 1 C–E) [3, 4].
Figure 1
Pre-procedural cardiac computed tomography analysis. A – Internal dimensions (ID) of Epic 31 mm valve; based on the Valve in Valve app – true ID = 26.5 mm. B – CT-ID, dimensions of the true lumen. C – Aorto-mitral angulation (AMA) suggesting low risk of LVOT obstruction (wider than 120°). D, E – Neo-LVOT calculation with virtual valve implantation showing an area above 2.0 cm2, which could increase the risk of LVOT obstruction
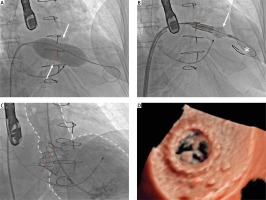
The procedure was performed via the transvenous route in general anesthesia under transesophageal echocardiography (TEE) guidance. After puncture of the interatrial septum in postero-inferior location and crossing through the degenerated valve into the left ventricle, dilatation of the intra septal channel was performed with a 12 × 40 mm peripheral balloon catheter. Predilation with a 22 mm balloon catheter was performed and upon inflation a clear waist was visible (Figure 2 A); therefore a 26 mm Sapien 3 (Edwards Lifesciences) valve was chosen. Despite the previous dilatation of the intra-septal channel the crossing with the delivery system showed some difficulty, but after changing to a Lunderquist Extra Stiff guidewire and some mild dilatation of the distal part of the Edwards balloon it was possible to obtain correct positioning (Figure 2 B). During rapid pacing the valve was implanted in a 20%/80% (atrium/ventricle) depth ratio and afterwards postdilated distally with an extra 2 ml of volume to obtain a wider, cone-shaped, ventricular end of the valve. In post-procedural angiography there were no signs of paravalvular regurgitation (Figure 2 C), and TEE showed good expansion of the implanted valve (Figure 2 D).
Figure 2
Transcatheter mitral valve-in-valve implantation. A – Predilatation of a 31 mm Epic bioprosthesis with a 22 mm balloon catheter. The waist is clearly visible (white arrows). B – Edwards SAPIEN 3 26 mm delivery. In order to facilitate the delivery, mild balloon inflation (white arrow) and changing to a Lunderquis Extra Stiff guidewire (asterisk) was performed. C – Final angiography showing no residual paravalvular leak (PVL). Aorta and left ventricle marked with white dotted line; red dotted line – Epic 31 mm valve stent. D – Postprocedural photorealistic TEE (Philips Epic CVx) showing excellent prosthesis position and no PVL
In carefully selected patients TMVI is a forward-looking method enabling less invasive treatment for severe mitral prosthesis failure. With the use of proper implantation technique [5, 6] the procedure is safe and feasible via the transfemoral venous route. The most crucial part of planning the procedure, besides screening for possible LVOT obstruction, is valve sizing. Given the possibility of late valve migration, undersizing should be avoided in most cases. In those situations the true ID should be chosen as the proper diameter to size the valve. In cases with extensive hypodense pannus formation or calcifications within the bioprosthetic valve an alternative may be used. Although controversial, balloon sizing may prove useful to determine the strength and flexibility of additional tissue found within the stent of the degenerated bioprosthesis, therefore providing new information on the appropriate transcatheter valve size for mitral valve-in-valve procedures.








