Introduction
The incidence of ectopic pregnancy (EP) is about 1.3–2.4%. About 6% of maternal deaths in the first trimester occur following ruptured EP [1].
The rising incidence of EP can be explained by the increased rate of ARTs, tubal surgeries, and improved diagnostic tools [2].
The fallopian tube (FT) is the site of oocyte fertilization. The migration of fertilized oocyte from FT to the uterus is mediated by FT muscles and cilia. Fallopian tube inflammation and/or dysfunction are implicated in oocyte retention and subsequent EP [3].
Several risk factors have been associated with EP [4]. Prior tubal surgery, prior EP, sterilization, intrauterine contraceptive device considered high-risk factors for EP. While infertility, current or prior pelvic inflammatory disease (PID), tubal pathology, smoking, and > one sexual partner are considered moderate risk factors for EP [4]. This report represents the treatment of left undisturbed tubal pregnancy with foetal cardiac activity using a two-dose MTX regimen.
Case report
A 35-year-old woman, G4, P3, pregnant 7 weeks + 2 days, presented on 2 October 2021 with left iliac pain, after positive pregnancy test (last menstrual period was on 16/8/21), and initial β-human chorionic gonadotropin (β-hCG) 3614 mIU/ml (Table 1).
Table 1
The β-human chorionic gonadotropin levels of the studied woman
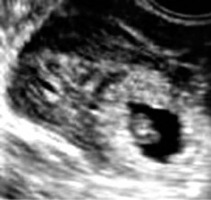
Transvaginal sonography (TVS) showed an empty uterus with a well-defined left adnexal echogenic structure measuring 38×32 mm (left adnexal gestational sac – GS) with foetal pole (bagel sign) (Fig. 1).
Fig. 1
Trans-vaginal sonography shows a well-defined left adnexal echogenic structure (left adnexal gestational sac) with foetal pole (bagel sign)
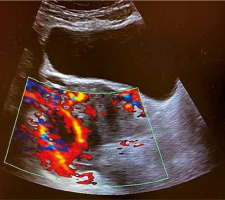
A departmental ultrasound with colour Doppler examination according to hospital policy showed foetal cardiac activity with circumferential Doppler flow around the GS (ring of fire) (Fig. 2).
Fig. 2
Colour Doppler examination shows circumferential Doppler flow around the gestational sac (ring of fire)
The diagnosis was left undisturbed tubal pregnancy with foetal cardiac activity. She refused the option of laparoscopic surgery and asked for medical treatment. She was informed that the medial treatment using methotrexate (MTX) may fail due to the presence of foetal cardiac activity and she may need more than one MTX dose. She was also counselled regarding the long hospital stay under observation and serial β-hCG assay.
After proper counselling, written consent, and departmental approval, she received the first dose of MTX on 2 October 2021 [75 mg MTX (50 mg/BSA)] at an initial β-hCG 3614 mIU/ml (Table 1).
The fourth day β-hCG after the first MTX dose increased to 5421 mIU/ml (in-spite of stopped foetal cardiac activity by ultrasound), while the seventh day β-hCG was 5055 mIU/ml [< 15% decrease of β-hCG (6.75%)]; therefore, she was given a second MTX dose.
The fourth day β-hCG after the second MTX dose was 3851 mIU/ml, and the seventh day β-hCG was 2218 mIU/ml [> 15% decrease of β-hCG (42.4%)] (Table 1).
Therefore, she was discharged home for follow-up in the outpatient department (OPD) using a weekly β-hCG level, after satisfactory ultrasound findings, which showed decreased GS size to 18×14 mm with minimal Doppler flow around.
The second week β-hCG after the second MTX dose was 292.4 mIU/ml, the third week β-hCG was 77.84 mIU/ml, and the fourth week β-hCG was 18.58 mIU/ml (the ultrasound showed complete disappearance of the GS).
The patient did not attend follow-up for 2 weeks then the seventh week β-hCG after the second MTX dose came as 0.100 mIU/ml (normal value 0.0–10.0 mIU/ml) (Table 1).
Written informed consent and departmental approval were obtained to publish the studied woman’s data as a case report.
Discussion
Suspicion of EP begins after a positive pregnancy test and absence of intrauterine gestational sac (IUGS) by ultrasound (pregnancy of unknown location – PUL) [1, 5].
The diagnosis of PUL changed to intrauterine pregnancy (IUP) in 30% of cases after identification of IUGS by ultrasound, and the majority of PUL (70%) changed to either miscarriage or ectopic pregnancy (EP) [1].
A retrospective review of PUL conducted by Morse et al. [6] suggested ≥ 35% β-hCG rise in 48 hours to diagnose IUP [6]. A β-hCG rise < 35% in 48 hours has 80.2% overall accuracy in predicting EP [1].
Visualization of an adnexal GS as an echogenic structure with yolk sac and/or embryonic echo (bagel sign) with circumferential Doppler flow (ring of fire) without IUGS is highly suggestive of EP [1].
If the visualized adnexal GS is round and moves separately from the ovary (blob sign) [4]. The blob sign has > 90% PPV in diagnosing EP in women with positive β-hCG and no definite IUGS [7].
A systematic review by Crochet et al. [8] found that 88% of tubal-EPs were diagnosed by absent IUGs and presence of adnexal mass during TVS.
Medical treatment of EP using MTX is more cost-effective than surgical treatment, with a similar success rate [9]. Methotrexate is a dihydrofolate reductase inhibitor, which targets the rapidly dividing trophoblasts [10].
Indications of MTX treatment in EP include a haemodynamically reliable and vitally stable patient, pre-treatment initial β-hCG 1500–5000 mIU/ml, ectopic size < 4 cm, no foetal cardiac activity, no concomitant IUP, and no known sensitivity to MTX [1].
The presence of foetal cardiac activity, β-hCG level > 5000 mIU/ml, an ectopic mass size > 4 cm, or unreliable patient are relative contraindications for use of MTX in the treatment of EP [1].
Vitally unstable women, heterotopic pregnancy (EP with viable IUP), elevated liver enzymes, high serum creatinine (≥ 1.5 mg/dl), low total leucocyte or platelet count (< 1500 mm3 or < 100.000 mm3, respectively), moderate to severe anaemia, breastfeeding, active pulmonary disease, or peptic ulcer, and sensitivity to MTX are the absolute contraindications of using MTX in treatment of EP [1].
The methotrexate dose is 50 mg/m2 intramuscularly, and the β-hCG should be checked on days 4 and 7 following the MTX dose.
The success rates of MTX are high with low initial β-hCG levels [4]. A meta-analysis showed that the success rate with a single MTX dose was 94.4% when the initial β-hCG level was between 1000 and 1999 mIU/ml, and 81.1% when the initial β-hCG level was between 10.000 and 15,000 mIU/ml [11].
The two-dose MTX regimen was proposed by Barnhart et al. [12], with 87% success rate and minimal side effects.
A retrospective study reported an 87% success rate for the single MTX dose regimen at an initial β-hCG of 4801 mIU/ml compared to a 90% success rate for the two-dose MTX regimen at an initial β-hCG of 4278 mIU/ml [13].
Decreased β-hCG by ≥ 15% on day 7 indicates successful MTX treatment, while an increase of β-hCG level or < 15% drop on day 7 indicates a second MTX dose [1].
Regardless of which MTX regimen is used, if the β-hCG does not decline adequately (< 15% on day 7) after the last MTX dose or it increases, surgical management should be considered [7].
If β-hCG decreases by ≥ 15% on day 7 after the last MTX dose, no further action is required, and the β-hCG should be monitored weekly until it reaches the non-pregnant level [1].
The studied woman received two-dose MTX because the β-hCG decreased by < 15% (6.75%) on day 7 after the first MTX dose (from 5421 to 5055 mIU/ml on day 4 and 7, respectively). The second MTX dose was successful and the β-hCG decreased by > 15% (42.4%) on day 7 after the second MTX dose (from 3851 to 2218 mIU/ml on day 4 and 7, respectively). Therefore, she was discharged home for follow-up in OPD using weekly β-hCG.
The second week β-hCG after the second MTX dose was 292.4 mIU/ml, while, the third week β-hCG was 77.84 mIU/ml, and the fourth week β-hCG was 18.58 mIU/ml. The patient did not attend for follow-up for 2 weeks, and then the 7th week β-hCG after the second MTX dose was 0.100 mIU/ml (normal value 0.0–10.0 mIU/ml).
The β-hCG usually returns to normal, non-pregnant level within 2–3 weeks [1] but can take up to 8 weeks in women with higher initial β-hCG [7–10].
Conclusions
The foetal cardiac activity is one of the relative contraindications but not an absolute contraindication of using MTX in the treatment of undisturbed tubal EPs. The two-dose MTX regimen can be used as an effective treatment option for undisturbed tubal EPs < 4 cm in size, with initial β-hCG < 5000 mIU/ml, and positive foetal cardiac activity in special situations (i.e. refusal of surgery) after proper counselling.











