Summary
In this revised version of the manuscript we provide data concerning the possibility not to use contrast medium during Magnetic resonance imaging examination of patients qualified for venous intervention due to advanced symptoms of post-thrombosis syndrome. Such modification of the examination protocol helps to reduce the cost of examination and reduce the patient’s risk linked with use of contrast medium. On the other hand, we found that intravascular ultrasound examination is necessary during venous intervention.
Introduction
Post-thrombotic syndrome (PTS) is the most significant of the long-term complications of deep vein thrombosis (DVT) [1]. More than 10 years ago, the possibility emerged of treating this clinical disorder by stenting the iliac and femoral veins [2]. It is recognized that preoperative diagnosis of the cause of iliac vein narrowing or occlusion (e.g. thrombus, compression), primary vein diameter, length of lesion, and localization of the stent landing zone can have a significant effect on the outcome of venous interventions, because this information is important for the selection of venous stents of the appropriate diameter and length. That information can potentially be obtained from duplex ultrasonography (DUS), conventional X-ray venography [3], computed tomography (CT) or magnetic resonance imaging (MRI) [4–6].
Contrast-enhanced magnetic resonance venography (MRV) has established clinical usefulness in the diagnosis of acute DVT [7, 8], even in pregnant women [9], and the determination of the age of a thrombus (acute, evolving or chronic) [10]. However, the use of a contrast medium not only prolongs the examination and increases the cost of the procedure, but may also be potentially harmful for patients, especially those with kidney failure. Therefore, several imaging techniques were recently proposed to avoid the use of a contrast medium without a reduction in the quality of MRI. The terminology used to describe the MRI data sequencing is different for the individual manufacturers of MR scanners (e.g. Philips, GE, Siemens, Hitachi, Toshiba). Examples of non-contrast-enhanced MRI (NCE-MRI) are as follows: time-of-flight (TOF) [11, 12], phase contrast angiography (PCA), non-contrast angiography (e.g. TRACE, NATIVE, VASC, FBI) [7, 12], volume-interpolated T1-weighted three-dimensional gradient echo with fat suppression (e.g. LAVA, VIBE, THRIVE, TIGRE) [13], balanced gradient echo (e.g. BFFE, BTFE, CISS, TRUE FISP, FIESTA, BASG, TRUE SSFP) with or without fat suppression [10, 14], ultra-fast T1-weighted three-dimensional gradient echo (e.g. MPRAGE, 3D TFE, 3D FGRE, 3D fast SPGR) [15–17], T1-weighted three-dimensional spin-echo with fat suppression (e.g. 3D TSE SPAIR) [18], T1-weighted three-dimensional multi-phase gradient echo (e.g. Dixon, mDixon, IDEAL, LAVA-FLEX), three-dimensional two-point Dixon gradient recalled echo with magnetization preparation and T2 preparation [19], and T1-weighted three-dimensional black-blood turbo spin-echo techniques [17].
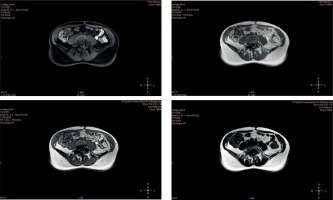
New sequences, such as the Dixon method used in this study, provide excellent resolution in soft tissue imaging, and allow tracing of the course, structure and size of vessels, as well as the potential cause (e.g. external vein compression) and location of the stenosis or obstruction [11, 19]. The Dixon method is an MRI sequence based on chemical shift and designed to achieve uniform fat suppression. It has been gaining popularity as it has some advantages over other fat suppression techniques (LAVA, VIBE, THRIVE, TIGRE). The main benefit of this sequence is to provide four sets of images during a single acquisition (in phase, out of phase, water only, fat only) for more details and diagnostic information (Figure 1). Dixon-based fat suppression can be very effective in areas of high magnetic susceptibility, where other techniques fail. Compared to the other NCE-MRI, such as time-of-flight (TOF), phase contrast angiography (PCA), or non-contrast angiography (TRACE, NATIVE, VASC, FBI), the Dixon sequence has a thinner layer and shorter scan time, and therefore the probability of moving artifacts is much lower. This technique, unlike the others, does not require the use of electrocardiogram (ECG) gating, which shortens the examination time, reduces general costs and protects against the loss of good quality of examination when the patient has cardiac arrhythmias [19]. If the vessel has an oblique or curvy course, the sequences mentioned may show a lower intensity of the signal that can be misconstrued as segmental narrowing of the vessel [11].
However, it is not known whether this post-processing MRI technique could be useful in the selection of an appropriate stent diameter and length. Therefore, intravascular ultrasonography (IVUS) is still considered the gold standard diagnostic tool for venous intervention, as it helps indicate the landing zone for a stent and the appropriate choice of stent diameter. However, IVUS catheters are expensive, so these examinations increase the cost of the procedure. For this reason, we initiated this study to answer two questions: (1) whether NCE-MRI could replace conventional contrast-enhanced MRI (CE-MRI) in the preoperative evaluation of patients with PTS, as it allows similar data about iliac and femoral vein section areas (VSAs) to be obtained. If the answer to the first question is yes, this might allow contrast infusion during MRI to be discontinued and thus reduce both patient risk and the total cost of this diagnostic procedure; and (2) whether the diameter of ipsilateral and contralateral iliac and femoral veins determined by CE-MRI and NCE-MRI is related to the respective ipsilateral VSAs calculated by IVUS examination. If the answer to the second question is yes, this might be a basis for discontinuing the use of IVUS in patients with a preoperative MRI evaluation and help decrease the total cost of venous interventions.
Material and methods
Patients
The research work included a group of 18 consecutive patients (13 female and 5 male), between 27 and 55 years of age, with a diagnosis of PTS, who qualified for venous intervention. The period between the date of an acute DVT episode and the intervention ranged from 5 to 8 years, and the average Villalta score before the intervention was 15 (range: 12–18). All patients were fully hydrated before the examination to avoid the effect of dehydration on VSA size [20].
Magnetic resonance imaging method
All the patients were imaged at 3.0 T (Philips Ingenia, manufactured in 2015) using a 16 channel anterior coil. The axial Dixon sequence was performed with the following parameters: scan type: imaging; scan mode: 3D; scan technique: FFE; contrast enhancement: T1; acquisition mode: Cartesian; Echoes: 2; TR/TE1/TE2: 3.6/1.3/2.3 ms; halfscan: no; RF Shims: adaptive; slice thickness: 3.5 mm; slice gap: 1.75 mm; phase encoding: AP; FOV: 400 mm (RL) × 280 mm (AP) × 220 mm (FH); acquisition matrix: 256 × 165; reconstruction matrix: 432; acquisition voxel size: 1.6 mm (RL), 1.7 mm (AP), 3.5 mm (FH); recon voxel size: 0.93 mm (RL), 0.93 mm (AP), 3.5 mm (FH); flip angle = 10°; acceleration factor (SENSE): P reduction (AP) = 2, S reduction (FH) = 1.4; number of signal averages (NSA): 1; SAR mode: high; PNS mode: high; gradient mode: maximum; reconstruction mode: real time; CE profile order: linear; total scan duration/breath hold = 14 s; mDIXON images (post-processing): IP (in phase), OP (out of phase), W (water only), F (fat only).
The study protocol consisted of an axial pre-contrast Dixon sequence, intravenous contrast injection, and the same Dixon sequence obtained post-contrast, 30, 60, and 300 s following administration of the contrast injection. The Dixon sequences consisted of four sets of images: water only, fat only, in phase and out of phase. During the examination, the gadolinium contrast was administered from an automatic syringe at a flow rate of 2 ml/s with a dose of 0.2 ml/kg of the patient’s body weight. Maximum intensity projection (MIP) images were created from the post-contrast Dixon sequences.
Magnetic resonance image analysis
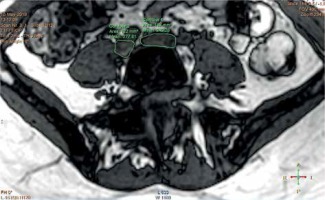
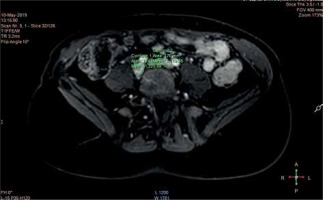
Using the control panel of the MRI scanner, we measured the VSA on the right and left sides (common iliac vein, external iliac vein, common femoral vein). The calculations were made using the technique of drawing the region of interest (ROI) outlining the shape of a given vessel, and the chosen VSA was measured automatically by the computer (mm2) (Figures 2, 3). The average diameter of the vessel was then calculated using the following formula: vein diameter (mm) = 2 × (VSA/3.1415)–0.5.
Conventional venography/venous intervention
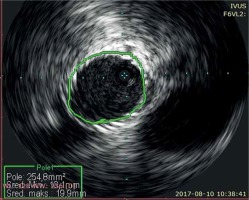
Conventional venography was performed by popliteal or jugular access in 15 subjects (10 female and 5 male) during venous intervention using a standard low-osmolar iodine-containing contrast medium. In all 15 patients, occlusion or severe narrowing of the common and/or external iliac vein found in MRI was confirmed. After restoring vein patency in all the patients, vein angioplasty was performed using a 12–14 mm high-pressure dilatation balloon (Atlas Gold, produced by BARD). IVUS imaging using a VULCANO device was then performed for every patient to determine the cause of iliac vein narrowing (e.g. external compression by an artery or tumor), the landing zone for the stent (i.e. site of an unaltered vein wall), and the diameter of the target vein for selection of the venous stent size. The last determination was calculated using the same method as with the MRI of drawing the ROI (Figure 4). Following placement, all stents were post-dilated with high-pressure balloons of the same diameter. IVUS and venography were performed after stent dilatation. Appropriate stent dilatation in IVUS and the disappearance of collateral veins in conventional venography were recognized as indicators of the technical success of the procedure.
Bioethics
The investigation was conducted in compliance with the Declaration of Helsinki for medical research, after receiving permission from local Bioethics Committee KB 291/2016.
Statistical analysis
Statistical analysis was conducted using licensed versions of the statistical software Statistica (a data analysis software system), Dell, Inc. (2017), version 13. The normal distribution of the study variables was checked using the Shapiro-Wilk test. The results were mainly presented as the median ± interquartile range (IQR), defined as the difference between the upper and lower quartiles, due to the non-normal distributions of the quantitative variables. The statistical significance of differences between groups was verified using the Wilcoxon non-parametric test and Kruskal-Wallis non-parametric ANOVA. The association between variables was checked using Spearman’s rank correlation.
Results
With the 18 patients for whom venous MRI was performed, we found statistically significant, but only small and clinically negligible, differences in cross-sectional areas of iliac and common femoral veins when we compared measurements obtained from MRI performed with (CE-MRI) and without (NCE-MRI) contrast enhancement (Table I). All the VSAs calculated from MRI with contrast enhancement correlated significantly with those calculated without contrast enhancement, with R values in the range 0.87–0.97 and p-values < 0.001.
Table I
Median cross-sectional area of iliac and femoral veins obtained during MRI with and without contrast enhancement
In three of the women who were enrolled, the suspected significant narrowing of iliac veins was not confirmed by MRI and, therefore, further analysis was based on measurements obtained for the 15 patients undergoing venous intervention (Table II). We found that ipsilateral VSAs determined using IVUS did not correlate significantly with the VSAs determined using either contrast-enhanced or non-contrast-enhanced MRI, for both the treated limb and the contralateral extremity. The R co-efficients ranged from –0.28 to 0.47, but the p-value for each correlation was > 0.05 (detailed data not presented). Clinically important differences were observed between VSAs determined using IVUS and MRI (Table II). These discrepancies were also observed when ipsilateral VSAs obtained using IVUS were compared with VSAs determined in CE-MRI and NCE-MRI, both for ipsilateral and contralateral (untreated, unaffected, healthy) extremities. This suggests that selecting a venous stent for venous interventions on the basis of iliac vein diameter determined using CE-MRI or NCE-MRI for an ipsilateral extremity (with a diagnosis of PTS) might be linked to a level of error of, on average, 27–60% (Table II).
Table II
Comparison of the median of the ipsilateral VSA of iliac and common femoral veins calculated in IVUS obtained in MRI with and without contrast enhancement for ipsilateral (post-thrombotic) and contralateral extremities
| Extremity/VSA | Common iliac vein | External iliac vein | Common femoral vein |
|---|---|---|---|
| Contralateral extremity: | |||
| VSA in CE-MRI [mm2] | 200.0; 160–275.0 | 160.0; 90.0–230.0 | 120.0; 90.0–140.0 |
| VSA in NCE-MRI [mm2] | 147.5; 113.1–195.0* | 150.0; 70.0–180.0 | 110.0; 70–130.0 |
| % difference in VSA calculated in CE- and NCE-MRI (%) | 8.3; 7.1–17.4 | 10.0; 3.2–22.2 | 5.9; 0.0–7.7 |
| Ipsilateral extremity: | |||
| VSA in CE-MRI [mm2] | 100.0; 51.5–172.5 | 90.0; 75–180.0 | 100.0; 70.0–135.0 |
| VSA in NCE-MRI [mm2] | 90.0; 41.0–180.0 | 80.0; 70–170.0 | 100.0; 70–135.0 |
| VSA in IVUS [mm2] | 201.1; 201–254.5* | 176.7; 153.9–201.1* | 133.5; 113.1–153.9* |
| % difference in VSA calculated in CE- and NCE-MRI (%) | 14.2; 8.9–17.5 | 6.7; 0.0–18.2 | 0.0. 0.0–8.3 |
| % difference in VSA between IVUS and CE-MRI (%) | 52.7; 14.2–74.4 | 41.5; –1.9 – 67.0 | 27.1; 5.8–42.5 |
| % difference in VSA between IVUS and NCE-MRI (%) | 60.2; 10.5–79.6 | 48.0; 9.6–65.2 | 35.9; 9.1–42.5 |
| % difference in VSA between ipsilateral IVUS and CE-MRI (obtained in contralateral extremity) (%) | 1.8; –36.8–20.4* | 11.9; –39.3 – 49.1* | –15.9; –41.5 – 20.4* |
| % difference in VSA between ipsilateral IVUS and NCE-MRI (obtained in contralateral extremity) | 27.9; 3.0–44.1* | 20.7; –13.7 – 60.4* | 26.7; 9.1 – 38.1* |
ipsilateral side – side on which venous intervention and IVUS examination were performed (data concern post-thrombotic limbs), contralateral side – VSA calculated for vein of healthy extremity, CE-MRI – contrast-enhanced magnetic resonance imaging, IVUS – intravascular ultrasonography, MRI – magnetic resonance imaging, NCE-MRI – non-contrast-enhanced magnetic resonance imaging, VSA – vein section area.
Discussion
In this study, we tried to determine whether MRV can be performed without contrast enhancement without reducing the clinical usefulness of the examination. Our evidence suggests a positive answer because we found a strong correlation between VSAs of iliac and femoral vessels when the results from CE-MRI were compared with those from NCE-MRI (Table I). Moreover, comparing CE-MRI and NCE-MRI showed only clinically insignificant differences in the VSA values obtained, which confirmed that MRI of iliac veins without contrast is sufficient for the proper diagnosis of iliac vein narrowing or occlusion (Table I). Our observations corroborate the study by Dillman et al. [19], who, in 64 patients (involving 73 limbs), confirmed the clinical usefulness of a novel 3D respiratory-triggered gradient recalled echo Dixon-based MRV. They found that this MRI technique provided high-resolution anatomical imaging of the vasculature of the neck, body and extremities without the need for intravenous contrast material or breath-holding. The clinical usefulness of NCE-MRI was also reported by Badowski et al. [11], who, in 18 patients with chronic renal failure, found that magnetic resonance pelvic venography without contrast injection made it possible to obtain good-quality images to assess vein patency and trace the course, structure and size of vessels, as well as the cause and position of a stenosis or obstruction. However, it should be underlined that Badowski et al. [11] used a 2D-ToF technique for vein exploration, which uses a completely different sequence from the Dixon method used in our research. The high clinical usefulness of NCE-MRI compared to standard venography was also reported by authors cited in the introduction section [7–9, 10, 14–18, 21].
Eliminating the need for contrast material has several advantages, including cost savings, removing the need for peripheral intravenous cannula placement, eradicating the risk of allergic-like contrast reactions, and minimizing any potential risks related to gadolinium retention in the body. The use of NCE-MRI allows the examination to be performed in patients with chronic renal failure [11]. Moreover, magnetic resonance examination without contrast enhancement does not depend on the timing of image acquisition relative to the injection of the contrast material, thereby preventing examinations that cannot be used for diagnosis due to images being taken too early or too late.
The second question we asked in this analysis was whether MRI could be helpful in the selection of an appropriate venous stent that would be adequate for the diameter of the treated vessel. To answer this query, we compared the cross-sectional areas of post-thrombotic veins determined ipsilaterally using IVUS during venous intervention (regarded as the gold standard) with VSAs of vessels calculated using CE-MRI and NCE-MRI in ipsilateral (post-thrombotic) and contralateral (healthy) extremities (Table II). We found that VSAs determined using IVUS and MRI differed on average by 27–60%, which suggests a potential for the mismatch of venous stent diameter. The potential for error might be reduced to 1.8–28% when VSAs obtained by IVUS are compared with those determined by MRI for a contralateral extremity (Table II). However, none of the Spearman’s correlations between the respective data for both the ipsi- and contralateral sides were statistically significant. Therefore, it seems likely that VSAs obtained by MRI are not suitable for selecting venous stent diameter and demonstrate the necessity to use IVUS during venous interventions. The advantage of IVUS is the precise assessment of pathologically changed venous vessels from the inside. IVUS during conventional X-ray venography is therefore a gold standard for implantation of stents of optimal size. In none of the papers available did we find a similar statement or comparison of the clinical usefulness of IVUS with MRI in patients with PTS who qualified for venous intervention. A possible explanation of the discrepancies between outcomes of MRI and IVUS examinations is that both CE-MRI and NCE-MRI were performed before restoration of vein patency, and only during MRI can the vein lumen be visualized [11]. Therefore, when the vein is occluded, the complete lack of signal make impossible to measure a VSA. Only when vein patency was restored during the intervention did an IVUS examination make it possible to trace the vein’s wall and to measure VSA (Figure 4). The other potential cause of mismeasurement of VSA in MRI is an oblique or twisted course of the vein [11]. Then, the intensity of the recorded signal may be poor or lost, which could suggest vein narrowing or occlusion.
Our study has some limitations. The most important limitation would seem to be its small sample size, as this reflects a relatively small number of venous interventions. In the majority of cited works, the sample size ranged from 8 [20] to 30 [4].
Conclusions
CE-MRV can be replaced by Dixon-based NCE-MRI in the preoperative evaluation of patients with PTS who qualify for venous intervention. However, CE-MRI and NCE-MRI performed both for ipsilateral and contralateral extremities are not sufficient for appropriate venous stent selection, and IVUS remains a necessary tool during venous interventions in iliac veins.