Introduction
Asthma is one of the most common chronic diseases and is associated with a variable airflow obstruction that is often reversible [1]. The clinical importance of a bronchodilator reversibility test (BT) is proven and the spirometry is the standard assessment technique for respiratory function according to standards [2]. The spirometry is the most widely-used lung function test in children. However, other methods assessing airway function that do not require complex respiratory manoeuvers are necessary. The most common methods for measurement of airway resistance that require less cooperation and less physical effort from the patient are plethysmography and the interrupter technique [3–6].
Aim
The aim of this study was to compare different applied definitions of airflow obstruction in children. We assessed the usefulness of forced expiratory volume in 1 s (FEV1), total specific resistance (sRtot) and interrupter resistance (Rint) in the bronchodilator reversibility test in clinical practice in children with asthma symptoms.
Material and methods
Study design
It was a prospective, real-life, non-interventional study. All children, aged 6–18, newly diagnosed with asthma according to GINA guidelines [1] in our Allergic Outpatient Clinic, between January 2015 and June 2017 and able to perform lung function tests, were included into the study. None of the patients was chronically treated with inhaled corticosteroids and/or leukotriene inhibitors. Subjects underwent a history taking, physical examination, spirometry, plethysmography and the interrupter technique. We decided to assess sRtot – one of the parameters which can be calculated from the specific airway resistance (sRaw) loop, since its sensitivity to partial obstruction of peripheral airways has been well established [7, 8]. When airway reversibility was being assessed, a bronchodilator was administered (400 µg of salbutamol) using a spacer. After 15 min all respiratory tests were repeated. A standard cut-off of 12% of predicted value for reversibility in FEV1 was employed [1]. Improvement in the pre-bronchodilator sRtot and Rint after administration of salbutamol ≥ 25% and ≥ 35% was assessed [2, 6, 9].
Patients were classified as atopic based on the history and skin prick testing (SPT). In patients unable to undergo skin testing (either on antihistamine drugs or SPT results were not consistent with the clinical symptoms), a serum specific IgE for a specific allergen was employed. In patients with seasonal allergy, all lung function testing was done out of the pollen season. The diagnosis of asthma was universally established by the allergists according to the standard definition of the disease in the latest GINA guidelines.
Ethics
The study was approved by the Medical Ethics Committee of the Medical University of Lodz. All parents or legal guardians gave their oral and written consent to the evaluation of data from medical documentation of their children.
The study was registered on www.ClinicalTrials.gov, with ClinicalTrials.gov ID: NCT01805635.
Detailed information on procedures
Detailed information on the methods employed is available in our previous papers [6, 10, 11] and in other authors’ publications [1, 2, 12, 13].
Statistical analysis
The statistical power of the sample was as follows: for FEV1 (134 complete records), the statistical power was 99.99%, for sRtot (124 complete records), it was 99.99%, and for Rint (133 complete records), it was 99.99%.
The investigated traits were described by way of measures of location – mean, median and quartiles, along with measures of dispersion – interquartile range, standard deviation, standard error of mean, 95% confidence interval, and minimum-to-maximum values.
Mixed-effects linear regression models were carried out in order to test the significance of changes in the investigated respiratory and plethysmography parameters before and after salbutamol. Mixed-effects logistic regression models were employed for binary variables. All the regression equations were controlled for the studied patients’ age and sex. Z-scores were also computed for Rint values and their changes during the study. A level of p < 0.05 was considered statistically significant. All the statistical computations were carried out by means of Stata/Special Edition, release 14.2 (StataCorp LP, College Station, Texas, USA).
Results
We included 135 children diagnosed with asthma into the analysis. There were 56 (41.5%) females. The studied patients’ mean age was 10.2 ±2.7 years. Of 135 children included into the study, all underwent spirometry, 122 of them plethysmography and 131 – the interrupter technique (not all parents gave permission to perform all tests as they were afraid of the children’s condition). Two children were not able to repeat spirometry after admission of β2-agonist. Perennial and/or seasonal allergy was diagnosed in all patients.
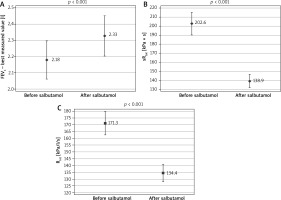
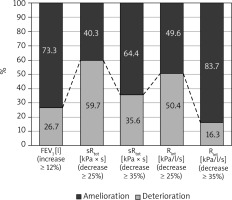
All investigated parameters changed statistically significantly after the bronchodilator administration in the examined patients (Table 1). Comparison of FEV1, sRtot and Rint results in BT in studied children is shown in Figure 1. The bronchodilator administration procedure yielded an improvement in FEV1 by at least 12% in 26.7% of the study participants. Concerning the plethysmography, sRtot decreased ≥ 25% in 59.7%, and ≥ 35% in 35.6%, while Rint ≥ 25% in 50.4% and ≥ 35% in 16.3% of children (Figure 2). The absolute change (decrease, to be precise) in Rint was 38.86 kPa/l/s. The absolute change in its z-score was 0.0538 (that is, as a result of 0.7917 – 0.7379). The logistic regression confirmed that the FEV1 was not so useful in diagnosis of asthma as the remaining two assessments, taking into consideration that all study participants had been correctly diagnosed with asthma (p < 0.001). The differences between the sRtot and Rint were not statistically significant in this context (p = 0.215).
Table 1
Descriptive statistics for selected measures of espiratory function in the studied patients before and after bronchodilator (salbutamol)
Discussion
We assessed usefulness of sRtot and Rint in the bronchodilator reversibility test alternative to FEV1 in standard clinical practice in children with asthma symptoms in the allergy out-patient clinic. The results indicated that sRaw and Rint performed better in a bronchial reversibility test taking into account that asthma was diagnosed in all participants in the asthma diagnostic process. Studies that have investigated the effect of bronchodilation on sRaw in young children have demonstrated reversibility with 500 µg of inhaled terbutaline (equivalent to 200 µg of salbutamol) [9, 13]. We administered 400 µg of salbutamol according to recommendations of ATS/ERS guidelines [2] and decided to use a spacer to avoid problems using a metered-dose inhaler (MDI) alone. Dose selection was supported by observation of Visser et al., who found significantly greater mean FEV1 reversibility after inhaling 400 µg salbutamol compared to 200 µg [14].
Evidence indicates that even in children below 6 years of age, pulmonary function tests produce technically satisfactory measurements using tidal breathing, the interrupter technique and forced oscillation [15–17]. However, the clinical utility in the management of the individual child needs careful consideration. Also, in some asthmatics, bronchodilator tests can be negative; a possible explanation is that airway acidification does not affect pulmonary function [18]. Our study used spirometry, plethysmography and interrupter technique to demonstrate airway reversibility in children. There are limited studies comparing the above respiratory tests in paediatric population. Black et al. proved a good correlation between the interrupter technique and spirometry or plethysmography [16]. In another study, it was demonstrated that sRtot was more sensitive than FEV1, and Rint in detecting bronchoconstriction in normal subjects [19]. Nielsen and Bisgaard compared the results of three different lung function techniques in asthmatic children during measurement of the bronchodilator response [9]. They concluded that sRaw has the highest discriminative capacity and serves as the most sensitive method in assessing bronchodilator responsiveness. Additionally, they suggested three standard deviation (SD) units (corresponding to a change of 25%) as the optimal cut-off level for discriminating between healthy children and asthmatic children. In our previous study we showed that sRtot (cut-off level – change of ≥ 25% in the pre-bronchodilator sRtot) was more sensitive and specific in identifying children with reversible obstruction than spirometry [6]. Sonnappa et al. measured multiple-breath washout indices (lung clearance index, conductive ventilation inhomogeneity (Scond)) and specific airway resistance (sRaw) in stable wheezers and in healthy children. Significant bronchodilator reversibility was only observed in wheezers for Scond but in both wheezers and healthy controls for sRaw, which is undermining the discriminating capacity of this technique in young children [20]. The results of our previous real-life study on 6439 children showed the lack of importance of sRaw in asthma diagnosis in schoolchildren [10]. This could be possibly explained by its overly high variability in schoolchildren, or a weak relationship between baseline FEV1 and sRaw, which was previously reported [21]. In another study, we suggested that in children with asthma-like symptoms at risk of the delayed asthma diagnosis, the spirometry together with the plethysmography should be performed to prevent underestimation of reversibility of bronchial obstruction and to increase the likelihood of early asthma detection [11].
In summary, parameters measured in our study may be useful in the diagnosis of asthma. However, the diagnosis of asthma is a clinical diagnosis that does not depend on bronchodilator responsiveness which may or may not be present at any specific time of measurement, especially when asymptomatic. Moreover, the total airway resistance is greatly influenced by the large airways which have the greatest component of airway resistance whereas disease is primarily present in the smaller airways. But the clinical advantages for measurements of small airway function, although potentially more relevant and can be estimated from some spirometry measurements, are not established. Therefore, our study has some limitations. Measure of overall airway resistance like sRtot or Rint is very sensitive to central airway pathology but less sensitive to peripheral changes. Also intra-subject variability in resistance measurements is much higher (5–15%) in comparison to 3–5% in FEV1 [5]. Another concern is the cut-off point, as some literature sources suggest 40% and some may suggest up to 50% change as significant [5]. In our study we used the 25% and 35% cut-off for resistance change. Lower cut-off for significance may be the cause of the increase in the number of positive cases in resistance improvement as compared to significant FEV1 change in this study. Contrariwise the 35% cut-off for resistance change has less sensitivity and may lead to underdiagnosis of asthma. In our patients, sRtot decreased ≥ 25% in 59.7%, and ≥ 35% in 35.6%, while Rint ≥ 25% in 50.4% and ≥ 35% in 16.3% of children. The limitation of our study is the lack of the control group of healthy children. However data on healthy children were published in the past. A study done on healthy preschool children revealed mean bronchodilator-induced changes (% of predicted values) – 15% for inspiratory Rint and 12% for expiratory Rint [21, 22]. In another study, a 35% decrease in resistance after bronchodilation expressed as the percentage of predicted value allowed for separating children with and without asthma [23]. According to ATS statement, a bronchodilation test using the interrupter technique should be considered clinically significant when the decrease in Rint after bronchodilator exceeds within-occasion repeatability between two sets of measurements established in 30 to 50 subjects for each individual laboratory [4]. Further studies are needed to establish the cut-off value for a decrease in airway resistance beyond which bronchodilator response should be considered clinically significant.
Our study suggests the interrupter technique can be successfully used to measure the response to the bronchodilator in the asthma diagnostic process in children. The main advantages of the interrupter technique, as described by Child, are that it is an easy, cheap, non-invasive, effort-independent measurement, reproducible in children as young as 2–3 years old and helpful in assessing bronchodilator responsiveness [24]. According to Black et al. [16], the interrupter technique may have a role in assessing baseline airway function and response to therapy in children unable to perform reliable spirometry, and/or when the investigators wish to avoid the possible influence of forced manoeuvres on airway tone.
Conclusions
Our results suggest that sRtot and Rint may be useful parameters in the reversibility test in clinical practice in the asthma diagnostic process in children. The above parameters could serve as a reliable tool in the evaluation of children with asthma-like symptoms. Finally, our results call for other studies, with an adequate sample size, addressing the usefulness of sRtot and Rint in diagnostics of asthma in children, giving high quality evidence to incorporate the plethysmography and interrupter technique into standard guidelines for the management of asthma in children. In summary, there may be use of resistance measurement mainly in children who cannot do spirometry, but it does not replace FEV1 because of its intrinsic multiple issues. Also, some new methods should be applied for evaluation of airway obstruction in children, such as structured light plethysmography [25].