Introduction
Utero-cutaneous fistula is an extremely rare condition that should be considered when, after caesarean section delivery [1, 2] signs of skin inflammation and/or infection persist. The literature provides only a few cases [3–11]. Utero-cutaneous fistula consists of an abnormal communication between 2 structures: the anterior wall of the uterus and the abdominal wall. Among the various types of fistulae that could involve the uterus, it is the rarest; the most common are represented by utero-bladder [12, 13] or utero-bowel fistulae [6, 7, 14]. These kinds of complications have a significant physical and psychological impact on the patients [15, 16].
The causes include multiple caesarean sections, incomplete hysterorrhaphy [17], miscarriages, uterine cavity revision, retention of placental material after delivery, use of drains, post-operative infections, or injuries [2–9, 18]. Other documented causes of this rare occurrence are intra-uterine devices and endometriosis – one of the most frequent causes of pelvic chronic pain [19–22]. Because it is a rare complication and the literature contains so few clinical cases, the correct diagnostic and therapeutic approach is a challenge for the gynaecologist. We present a patient with 2 previous caesarean section deliveries, who has undergone this rare complication.
Case presentation
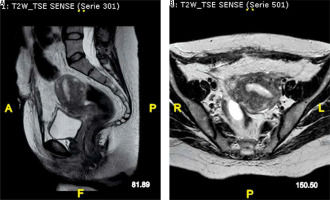
A 38-years old female, who had undergone a caesarean section 42 days earlier at another hospital unit, presented to our obstetrics and gynaecology emergency room with the chief complaints of fever (38.5°C), abdominal pain, and purulent discharge from the abdominal wall from 6 days. Her history included 2 previous term caesarean section deliveries, and a hysteroscopic polypectomy 2 years earlier. No IUD was inserted. No inflammatory pathologies or other noteworthy ones. No smoking or drug addiction. On the fourth day after the caesarean section, she was discharged from the hospital in good general condition. From the sixth day post caesarean section, she reported fever with a body temperature of 39°C for which she took an amoxicillin/clavulanic acid one tablet 2 times a day for 3 days on the recommendation of her general practitioner, then on the advice of the same doctor she switched the therapy with ciprofloxacin 500 mg one tablet 2 times a day for 6 days, and for the last 2 days clarithromycin 500 mg one tablet 2 times a day was added. From the day before admission to our gynaecology and obstetrics complex operating unit, on the advice of her gynaecologist, she began intravenous antibiotic therapy with piperacillin + tazobactam 4.5 mg one ampoule 3 times a day and intramuscular calcium nadroparin 0.4 IU one ampoule a day. She showed us an upper and lower abdomen computed tomography (CT) scan with contrast medium performed at the hospital where she did the caesarean section in which a fluid/super-fluid phlogistic collection was reported at the anterior wall of the uterus, which determined continuous solution of the uterine wall itself; it was also detected through fistulous with the anterior abdominal wall, in the hypogastric area, in the context of which further fluid/super-fluid phlogistic collection with hyperintense walls after contrast medium was detected. On examination: she was pale, but apart from mild tachycardia and fever (38.5°C) her vitals were stable. The abdomen was treatable but painful on superficial and deep palpation on all the upper quadrants, especially in the hypogastric area. Pfannenstiel scar with a continuous median solution of about 4 cm from which purulent fluid drains. Painless Douglas. Normal external genitalia of a woman who has never given birth vaginally. Regular vagina and cervix, uterus in puerperal involution 3 fingers below the transverse umbilical line painful when mobilized. Ovaries non palpable. Not atypical and no blood loss from external genitalia on vaginal exploration. In addition, a transvaginal ultrasound was performed: it represents a low-cost procedure that any gynaecologist is confident [23]. The exam showed a uterus with regular echo-structure and slightly increased morpho-volumetry, and thin endometrial rhyme. In the hysterotomy site, a mixed echogenicity area of about 4.8 × 3.2 cm, color score 1, probably attributable to granulation tissue, was present. Ovaries in place and bilaterally within the limits. Presence of modest free fluid flap in the Douglas pouch. Blood chemistry tests were performed in the emergency room to assess CBC, RCP, coagulation, and clinical chemistry. The WBC was 19.58 × 109/l, and no serious anaemia was found (Hb 10.8 g/dl). CRP was 22.44, and coagulation and clinical chemistry were within limits. It was decided to hospitalize the patient for suspected utero-cutaneous fistula and to set up a therapy based on amoxicillin 1 g one tablet two times a day, intravenous metronidazole 600 mg one ampoule three times a day and cefazoline 1 g one ampoule two times a day, intramuscular calcium nadroparin 0.4 IU one ampoule a day, acetaminophen as needed, and intravenous fluids. The next day, magnetic resonance imaging (MRI) with and without contrast, T1-, T2-weighted, T2 FAT-SAT, and THRIVE sequences of the lower abdomen and pelvic excavation were performed. The radiologist reported in the context of the anterior uterine wall an oval area of about 20 × 30 mm, hypointense in T1 with an inhomogeneous signal in T2, with blurred labrum of post-contrast enhancement suspected of abscess collection, which showed through hyperintense fistulous, extended for 30 mm and 16 mm of thickness, which ended in the subcutaneous area with an abscess joint (approximately 7 mm), without continuous solution with the skin. Below it, other via fistula with a thickness of 9 mm extended by approximately 34 mm was appreciated between the uterine wall and continuous solution with the external cutaneous planes of the interior abdominal wall (Fig. 1 A, B).
Fig. 1
Pre-surgery magnetic resonance imaging shows (A) uterocutaneous fistula (arrows) in the sagittal plane and (B) in the axial plane
During the days following the next blood chemistry check, haemoglobin was stable (10.6 g/dl), thanks to antibiotic therapy the WBC decreased but CRP remained high, and an increase in painful symptoms was registered in the abdominal area. It was thus decided to take the patient to the operating room after obtaining a specially drawn up informed consensus. Previous reports pointed out the laparotomic surgical excision of the fistula tract as the treatment of choice [7]. Only a few reports in the literature describe successful laparoscopic surgical management of the uterocutaneous fistula [24, 25], although evidence clearly shows the superiority of the minimally invasive surgery routes in terms of safety and efficacy in the case of benign gynaecological pathologies [26]. In this case, we opted for a liquid penetrant examination procedure: after preparation of the operating table, positioning of a Foley catheter, disinfection of the skin, and delimitation of the operating field with sterile sheets, a Pfannenstiel incision was performed on the scar from a previous caesarean section. The abdominal wall was opened in layers. The fistulous tract was highlighted. Peri-uterine fistulous tissue was severed. An intrauterine Foley was inserted as a guide to reconstruction of the anterior wall of the uterus, followed by double-layer hysterorrhaphy and thorough cleaning of the abdominal pelvic cavity. Haemostatic material was placed on the hysterotomy scar and careful treatment of the haemostasis was practiced. After counting needles, gauze, and surgical instruments reported by the nursing staff present in the operating room, the abdominal wall was carefully closed in layers, with an EBD of about 200 cc. The operating pieces were sent to the pathology laboratory for definitive histological examination. On microscopic examination multiple connective-adipose resections were observed characterized by large areas of fibrosis, zonal areas with necrotic-regressive phenomena, and widespread and intense chronic granulomatous inflammatory infiltrate, moderately active, with numerous giant cells of the “foreign body” type (sutures) and with partial aspects of the xantogranulomatosa type, with several foamy histiocytes. Areas of dilation and vascular congestion were also observed, associated with areas of haemorrhagic spread with some siderophages. The pathologist confirmed the surgical suspicion of utero-cutaneous fistula. After the surgery, the patient’s hospitalization continued for 7 days. Vital signs were carefully monitored 3 times a day, daily change of the dressing was done, and antibiotic therapy was continued until hospital discharge. At the time of discharge, the vital parameters and blood chemistry tests of the patient were normal, and the surgical suture was well adhered and in order.
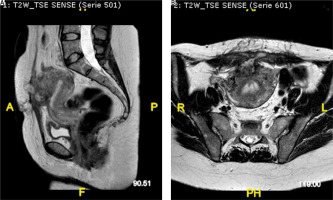
A month later the patient returned to our ward for a clinical suture check and an MRI. The radiologist reported the sub-umbilical surgical scar, and absence of sinus pathways between the anterior uterine wall and the abdominal wall (Fig. 2 A, B). The patient underwent further clinical checks in the following weeks; no signs of skin infection or recurrence of uterus-cutaneous fistulas were ever recorded.
Discussion
Although utero-cutaneous fistula is an extremely rare complication (fewer than 20 cases of utero-cutaneous fistula have been reported in the last 20 years worldwide up to now), it should be considered if, after caesarean section delivery, signs and symptoms of skin inflammation and/or infection persist.
The gynaecologist must therefore adequately investigate the cause of the inflammations and, if there is a suspicion of utero-cutaneous fistula, multiple diagnostic tools should be at his/her disposal: CT scan with intravenous injection of contrast medium and MRI can allow the identification of the fistula.
Furthermore, the internal fistulous opening may be visualized by means of hysteroscopy, whereas fistulography with the injection of the contrast material through the cutaneous fistulous opening allows confirmation of the connection to the uterus [4]. Treatment options include surgical excision of the fistula tract by means of an open surgical approach or minimally invasive surgery [24]. Conservative treatment with gonadotropin-releasing hormone agonist alone has been proposed also, with complete resolution of the fistula [27].