Since the first successful surgery using cardiopulmonary bypass (CPB) in 1953, there has been a constant improvement in the treatment of congenital heart disease (CHD) [1]. Advanced surgical and anaesthesia techniques, catheter interventions, comprehensive extracorporeal circulation (ECC) management and progress in postoperative care have all contributed to these results. Nevertheless, surgery for CHD is associated with a major insult to the cardiac muscle resulting in postoperative functional impairment of the heart. Sequelae of CHD surgery are a long-term burden and promote the development of cardiovascular and other morbidities. Heart failure, arrhythmia and hypertension are the most common consequences of congenital heart surgery (CHS) [2]. It has been reported that obstructive pulmonary disease was found to be the most common non-cardiovascular late morbidity after CHS; however, one should take into consideration neurological sequelae, especially epilepsy, psychiatric and endocrine disorders [2]. Low cardiac output syndrome (LCOS) is a major complication of cardiac surgery associated with significant morbidity and mortality. Its prevalence in paediatric patients remains high – nearly 10% of patients may develop LCOS after surgical correction of CHD [3].
Post-cardiac surgery myocardial damage can be a consequence of prolonged duration of cross-clamping or cardiopulmonary bypass, poor cardioprotection, systemic inflammatory response to cardiopulmonary bypass, direct myocardial injury, coronary or venous graft embolism and other complications of the procedure.
The troponin complex, which is a biomarker of choice for detection of myocardial injury, has been established as having prognostic value also following cardiac surgical procedures, mainly in adults and to a lesser extent in paediatric populations [4–6].
Its biochemical profile makes it a suitable tool in the early postoperative period. After myocardial damage, the rise of troponin T (TnT) is to be observed in the initial 3–6 hours. The maximal concentration is reached within 12–24 hours [7]. The following 40–48 hours are defined as a plateau phase with little alteration of TnT concentration. Subsequently, a gradual decrease of TnT concentration is observed; this phenomenon lasts 240 hours, depending on myocardial injury range and severity [7].
Further measurements of TnT concentration may provide a falsely elevated concentration of the biomarker in cases of impaired renal function, a phenomenon present in the adult population [8]. Troponin concentration has been found to have clinical applications, allowing in-hospital mortality, myocardial complications, cardiogenic shock, and arrhythmic complications to be predicted [6]. Gene-ral data which confirm the established fact of the existing correlation of troponin concentration with the severity of myocardial damage are continuously supplied; however, cut off values predicting adverse outcomes are still lacking.
TnT is a more cardiac-specific marker of cardiac muscle injury than CK-MB or myoglobin and its dynamic distinguishes well between acute coronary syndromes and chronic conditions [9]. Additionally, TnT measurements have been described as more consistent between laboratories because of the diversity of platforms that might play a role in comparative studies [8].
The aim of the study was to determine whether serum TnT levels measured preoperatively, and at 12 and 24 hours after CPB, can be used for prediction of the length of stay in paediatric cardiac ICU (ICU-LOS) and correlate with surgical complexity and postoperative course after CHD repair.
METHODS
Patient characteristics and data collection
We included 41 patients undergoing elective surgical repair of CHD with CPB in the Polish Mo-ther’s Memorial Hospital between August and December 2017. Approval of the Polish Mother’s Memorial Hospital Research Institute Ethical Committee was obtained (No. 23/2018).
The median age of the analysed patients was 37 months (range 14 days – 17 years). The group comprised neonates (n = 3; 7.3%), infants (n = 18; 43.9%) children 1 to 3 years old (n = 4; 9.8%), and aged 4 to 17 years (n = 16; 31.7%). The majority of patients (n = 28; 68.3%) were male, and the median body weight was 11.65 kg (3.4–66.0 kg). Twenty-three children (56%) suffered from complex heart disease, while 18 (44%) were treated for a simple lesion. Cyanotic heart defects were present in 80.5% of them. Types of defects are presented in Table 1.
TABLE 1
Types of congenital heart diseases
The following parameters were measured: Aristotle Basic Score (ABS) of the performed procedure [10], total time of CPB, total time of aortic cross-clamping (CC) and maximal Vasoactive-Inotropic Score (VIS) during treatment in the ICU. When multiple CPBs during the procedure were required, total time was used for analysis. ICU length of stay (LOS) was noted. Based on ABS assessment patients were grouped into 4 categories.
Aristotle Basic Score
ABS was designed and developed as an evaluation and comparison tool for congenital heart surgery. The project started in 1999 and is a consensus opinion of over 50 senior experts from 23 countries. It allows for a precise rating of the complexity of 145 congenital heart procedures, regardless of the centre and surgeon performing the procedure.
Three factors are considered: mortality potential, morbidity potential and anticipated technical difficulties of the procedure. ABS score ranges from 0.5 to 15 points and rates forms of performed surgery [11].
Troponin measurements
TnT concentration was measured in plasma samples collected at 3 time points: after induction of anaesthesia (t1), 12 hours after removal of CPB (t2) and 24 hours following CPB (t3). Blood samples were transferred directly to the in-hospital laboratory. The Elecsys Troponin T high sensitive assay (Roche Diagnostics) was used. The standard limit of detection was 5 pg mL–1. This assay is based on the recognition of epitopes located in the central part of the TnT protein by two monoclonal antibodies.
Anaesthesia protocol
Patients aged over three months received oral premedication (midazolam 0.5 mg kg–1) at 30 min prior to the theatre arrival [12]. Ketamine 2 mg kg–1 i.v. + midazolam in weight-adjusted dosage was used for induction of anaesthesia in neonates, while in older patients propofol or ketamine were used alternatively, depending on the patient’s haemodynamic status. Prior to intubation, fentanyl (1–3 µg kg–1) and pancuronium (0.1 mg kg–1) were administered, with boluses repeated during the procedure [6]. Sevoflurane/air/oxygen mixture was used for lung ventilation. Children below 1 year of age received methylprednisolone at a dose of 30 mg kg–1 before the surgical intervention [7].
Cardiopulmonary bypass protocol
Heparin in a dose of 300 IU kg–1 was administered prior to CPB. Activated clotting time (ACT) time was kept ≥ 480 s to provide anti-coagulation. Extracorporeal circulation (ECC) was commenced with a standard roller pump, membrane oxygenator and air-oxygen mixer. In hypothermic procedures, core temperature was reduced to 18–28°C by cooling with ECC and thermal mattress. All patients were treated with cold crystalloid cardioplegic solution. In babies younger than 12 months blood cardioplegia was used, with packed red blood cells added to the standard solution [14]. After surgery and removal of the aortic cross-clamp, the rewarming procedure was introduced. Protamine sulphate was administered for neutralization of heparin in a standard protamine to heparin ratio of 1.2 : 1.
Vasoactive–Inotropic Score
Patients received vasoconstrictive and inotropic medications according to their clinical condition. In the ICU the Vasoactive–Inotropic Score (VIS) values were calculated according to the following formula: VIS = dopamine dose (μg kg–1 min –1) + dobutamine dose (μg kg–1 min–1) + 10 × milrinone dose (μg kg–1 min–1) + 100 × epinephrine dose (μg kg–1 min–1) + 100 × norepinephrine dose (μg kg–1 min–1) + 10 000 × vasopressin dose (U kg–1 min–1). Maximal VIS was included in the analysis [15].
Statistical analysis
Quantitative variables were presented as median and range, qualitative variables as absolute values and percentage. Normality of TnT concentration distribution was assessed with the Shapiro-Wilk test. It was other than normal; thus we used the Friedman ANOVA to compare TnT concentrations between three time points, while in comparison of survivors vs. non-survivors the Mann-Whitney U test was used. Statistical relationships between TnT levels and the ABS score, times of CPB and CC, the VIS score and ICU-LOS were evaluated using Spearman’s rank correlation coefficient. The statistical significance was assumed for values of P < 0.05. The STATISTICA 12 software was used for data analysis.
RESULTS
The median Aristotle Basic Score of the analysed cohort was 8 points (3.0–14.5). Five patients belonged to the 1st difficulty level (1.5–5.9 points), 11 patients to the 2nd (6.0–7.9 points), 13 patients to the 3rd (8.0–9.9 points) and 12 to the 4th category (10–15 points). As 25 patients (60.9%) were classified as difficulty levels 3 and 4, the cohort should be considered as burdensome [4, 21]. Three patients died. The median ECC time in survivors was 110 min (range 43–182 min), shorter than in non-survivors (median 148 min). The median CC time was 49 min (10–111 min), the median length of stay in the ICU was 42.7 hours (12.6–307.7 hours), and the maximal median VIS score in ICU was 12.0 (range 0–65). The median initial TnT concentration was 15.5 pg mL–1, ranging from < 3 pg mL–1 (undetectable) to 391.2 pg mL–1. At 12 hours after ECC the median TnT levels were significantly higher: 860.5 pg mL–1 (range 187.0–3571 pg mL–1) and at 24 hours (t2): 595.6 pg mL–1 (121.6–5142 pg mL–1).
Troponin T concentration and ICU length of stay
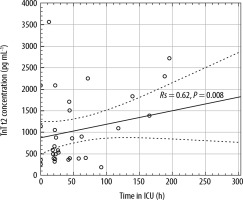
A strong positive correlation was found between TnT values at t2 and ICU-LOS, having a high Spearman’s rank correlation coefficient (Rs = 0.62, P = 0.008, Figure 1). A moderate correlation was found between TnT concentration 24 hours after CPB (t3) and LOS (Rs = 0.44, P = 0.018).
Troponin and complexity of the procedures
A correlation was found between postoperative TnT concentration and Aristotle Basic Score at t2 (Rs = 0.50, P = 0.001), but not at t3 (Rs = 0.48, P = 0.083). This might illustrate the association between surgery complexity and myocardial injury.
Troponin and duration of cardiopulmonary bypass
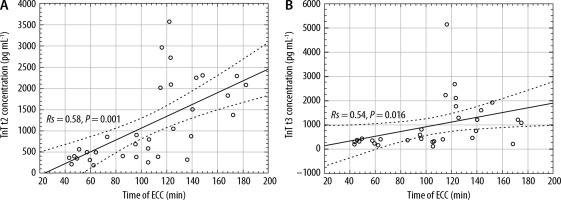
TnT concentration correlated significantly with total time of ECC at both t2 (Rs =0.58; P = 0.001; Figure 2A) and t3 (Rs = 0.540; P = 0.016; Figure 2B).
Troponin and duration of cross-clamping
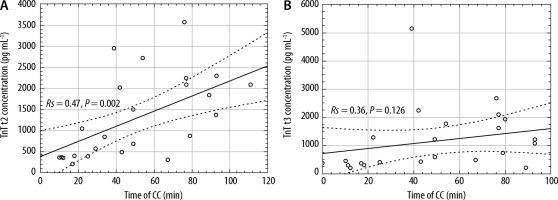
A positive correlation (Rs = 0.47, P = 0.002; Figure 3A) was found between TnT plasma level at the t2 timepoint and duration of aortic cross-clamp. This association was not present at t3 (Rs = 0.36; P = 0.126; Figure 3B).
Troponin and inotropic support
The median maximal VIS was 12 (range: 0–65). Only four (9.75%) patients did not require any inotropic support during hospitalization in the ICU. There was a significant correlation between TnT and maximal VIS score 12 hours after CPB (t2, Rs = 0.42; P = 0.001), with no correlation found at point t3 (Rs = 0.31; P = 0.254).
Troponin and outcome
Table 2 presents differences in TnT levels at three time points between survivors and non-survivors.
TABLE 2
Difference in troponin T (TnT) levels at three time points between survivors and non-survivors. Results are presented as median (range)
DISCUSSION
It has been proven that troponin T and troponin I change in similar ways following cardiovascular surgery [4]. The serum troponin T concentration was chosen for analysis due to its specificity. As shown in a 2017 metanalysis, when compared to TnI, it had a nominally higher associated hazard ratio for future cardiovascular events (HR 1.60 vs. 1.36; P = 0.171), and was significantly more strongly associated with fatal cardiovascular diseases outcomes (HR 1.99 vs. 1.56) [17].
Additionally, the diagnostic accuracy of TnT has been proven in children with CHD. Tarkowska et al. [18] reported a difference in TnT concentrations between newborns suffering from CHD and healthy individuals with a significant correlation between TnT concentration and haemodynamic significance of CHD in the studied newborns. However, they reported no correlation between TnT and type of heart disease or clinical symptoms of heart failure.
We have shown that TnT may be of predictive value when assessing the time of hospitalization in the ICU and the requirement for inotropic support in paediatric patients operated on for correction of CHD. It also correlates with the complexity of the procedure and duration of CPB and aortic cross-clamp.
A wealth of literature describes troponin reference values and their role in outcome prognosis following cardiac surgery, both in adult and paediatric populations [4–6, 19]. Some authors suggest that TnT concentration in children is a powerful marker of outcome after cardiac surgery [20, 21].
Mildh et al. [22] found that TnT level assessed shortly after ICU admission may allow one to predict postoperative course and complications. It has also been shown that TnT elevation in children reflects myocardial damage not only after the surgery, but also after chemotherapy with cardiotoxic doxorubicin [23]. The difficulty and complexity of performed surgery, cardiopulmonary bypass and cross-clamp times, surgical and reperfusion injury have all been found to cause major postoperative troponin release [19, 24, 25]. Troponin concentration has been found to predict myocardial complications such as arrhythmias, cardiogenic shock and in-hospital mortality [26]. On the other hand, Momeni et al. [27] did not find a correlation between troponin concentration and midterm mortality after heart surgery and do not recommend routine troponin monitoring. Limited predictive value of higher troponin T concentrations for the postoperative course was reported by Christmann et al. [28]. These authors, however, associate biomarker rise with the long and complicated operative course and this finding corresponds well with ours. Some reports indicate that certain levels of troponin T may be associated with a significant risk of a fatal outcome at day 30 [29]. As our study did not follow patients after discharge from the ICU, such a phenomenon could not be observed. When assessing TnT concentrations several precautions should be taken into consideration. Numerous conditions, cardiac and non-cardiac, e.g., renal failure, pulmonary hypertension and embolism, and septic shock, are associated with altered TnT [30, 31]. Gupta-Malhotra et al. [32] observed higher levels of troponin in neonates than in older children, while no differences in operation protocol were found.
Even though a clear tendency can be seen in our study, a larger sample is required for confirmation. Data which provide cut-off values of TnT predicting adverse outcomes are very limited. It has also been found that high troponin concentrations are not necessarily related to fatal outcomes [26]. Additionally, it is worth noting that the interpretation of troponin values after paediatric cardiac surgery without studies as ours is, in fact, not so apparent. The observed values, e.g., median values at t2 860.6 pg mL–1 (860.6 ng L–1) in the survivors group, reach high concentrations. Such TnT levels are observed in adults suffering from ST-elevation myocardial infarctions but, contrary to the adults, in the paediatric population, they are not necessarily followed by serious complications.
Limitations
The study group heterogeneity, which is determined by the inclusion of patients suffering from numerous complex congenital heart diseases as well as simple heart lesions, may limit the applicability of our results. The crucial limitation we would like to accentuate is the inclusion of neonates together with older children in the studied cohort. The infants subgroup (aged 0–12 months) comprised 21 patients, which constituted 51.2% of all included individuals. Guidelines concerning the interpretation of troponin T in this group of patients are still lacking. Numerous cut-off points exist, but no consensus regarding a final value has been reached [33]. Moreover, the diversity in terms of the age of the patients (spanning from 14 days to 17 years) should also be considered a limitation. Cohort augmentation should enrich collected data significantly – precise scope on discrepancies in tendencies present between survivors and the non-survivors group would benefit in suitable analysis concerning survivors only. What is more, further data concerning the occurrence of postoperative complications such as transient reduction of cardiac output would be highly beneficial.