Introduction
Cutaneous basal cell carcinoma (BCC) is the most common malignancy in the white population; according to the American Cancer Society, in 2006 about 3.5 million people were treated for non-melanoma skin cancer, mostly BCC [1, 2].
The main carcinogenic factor is ultraviolet light (UV), which explains why most tumours are located on sun-exposed sites. At the genetic level, the primary driver is the activation of the Hedgehog (Hh) signalling pathway with inactivating mutation of PTCH1 and activating mutations of SMO. Multiple BCCs are often associated with genetic disorders such as nevoid basal cell carcinoma syndrome (NBCCS, Gorlin syndrome) due to germline mutations in PTCH1 and, less frequently, in PTCH2 and SMOH. Also, P53 and Ras mutations play a role in the pathogenesis of sporadic BCCs, while SUFUH alterations are restricted to just a few cases [3]. Another genetic disorder is xeroderma pigmentosum due to germline mutations in DNA repair genes and albinism [4, 5]. The gold standard treatment for BCC is surgical excision with histological control of excision margins, but other locoregional approaches such as radiotherapy, cryotherapy, and photodynamic therapy are available. Radiotherapy (RT) may be considered a primary treatment in patients who are not candidates for surgery (locally advanced disease, comorbidities, or decline surgery) or when curative surgery is not recommended for poor aesthetic outcome.
Exposure to ionising radiation induces BCC progression due to radiation-induced DNA damage and subsequent abnormal cellular responses, tumour suppressive pathways, or activation of oncogenic signalling [6, 7].
Vismodegib (GDC-0449) is an oral molecule, inhibitor of thesmoothened receptor in the Hh pathway, approved by the FDA in 2012 for the treatment of patients with metastatic or locally advanced BCC inappropriate for surgery or radiotherapy, after its efficacy and safety were proven by a multicentre, international, phase 2 study that showed objective responses in 30% of patients with metastatic BCC and in 43% of patients with locally advanced BCC, with a median duration of response of 7.6 months in both cohorts [8–12].
Case report
In September 1994 a 22-year-old man had histological diagnosis of Hodgkin lymphoma, nodular sclerosis stage IIA, for laterocervical, supraclavear, and mediastinal nodes at the University Hospital of Pisa.
From October 1994 to February 1995, the patient was treated with 25 mg/m2 doxorubicin, 10 IU/m2 bleomycin, 6 mg/m2 vinblastine, and 375 mg/m2 dacarbazine-based chemotherapy for four cycles. Subsequently a CT scan showed complete response of disease, and the patient underwent external beam radiotherapy (EBRT) until May 1995.
EBRT used 18-MeV photons and was delivered with three-dimensional conformal radiotherapy. The target clinical volume included laterocervical, supraclavear, and mediastinal nodes. The total RT dose was 30 Gy in four weeks in daily fractions of 1.5 Gy, followed by EBRT boost on mediastinal nodes up to a dose of 10 Gy in daily fractions of 2 Gy. Subsequently, the patient underwent EBRT on lomboaortic nodes; RT total dose was 30 Gy in four weeks in daily fractions of 1.5 Gy.
Acute and late toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0 [13]. Adverse events were grade 2 nausea and vomiting, grade 1 diarrhoea, grade 1 dermatitis, and neutropaenia.
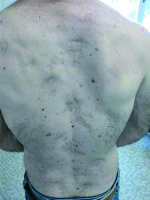
Subsequently follow-up showed no evidence of disease, until March 2012 when a clinical examination showed multiple basal cell carcinomas occurring in the field of prior radiation. No other lymph-node or distant metastases were detected. Hence, the patient underwent several local excisions of recurrent periodically cutaneous malignancies (Fig. 1). Histopathological assessment showed cutaneous basal-cell carcinomas. No mutations of Hh pathway or other genes were found, and no NBCCS was diagnosed.
In February 2018, after exhaustive discussion between the plastic surgeon, oncologist, and radiotherapist, the patient began therapy with vismodegib at standard dose 150 mg orally daily. Adverse events were minimal. The patient reported dysgeusia and subsequent weight loss. We did not register any significant changes in the patient’s haematological tests.
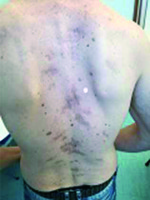
The patient was treated with vismodegib for 10 months and followed up until July 2019. After three months he underwent skin examination with negative biopsy, and after six months a CT scan was performed, with no pathologic evidence of disease (Fig. 2).
Discussion
BCCs are the most frequent post-radiation cutaneous malignancies, and they occur many years after radiotherapy; also, if low incidences are observed, a patient’s self and medical skin examination is recommended [14, 15].
A lower radiation dose at the edge of the radiation field may be not be able to kill all cells but may cause cell damage and genetic mutations. Disorganised reparative proliferation may primarily cause tumour development, and a low to intermediate dose range may significantly increase the risk of second cancer.
Moreover, an Iranian study showed that radio-induced and radio-recurrent BCC were more aggressive, difficult to completely eradicate by surgery, often with more risk of recurrence [16]. A small clinical trial showed promising results for vismodegib in treating multiple BCCs induced by radiotherapy; in eight patients who underwent radiotherapy four patients had partial response, three had stable disease, and one did not have a continuous follow-up at 34 weeks [17].
A 39-month update of a phase 2 multinational trial found an increased objective response rate for advanced BCC patients treated with vismodegib; 48.5.% in metastatic (m) disease and 60.3% in locally advanced (la) disease. Median OS was 33.4 months in the mBCC cohort and not estimable in the laBCC cohort. Of the thirty-three deaths (31.7%) reported, none were related to vismodegib [18].
In STEVIE, a multicentre trial of 499 advanced/metastatic BCC patients with a long follow-up treated with vismodegib, 36% had adverse events, 14% had disease progression, and 10% permanently interrupted this treatment. Most adverse events were grade 1 or 2, but 21 treatment-relate deaths were reported. The overall response rate was 66.7% in laBCC and 37.9% in mBCC [19].
Also supporting the efficacy and the safety of vismodegib, in an open-label multicentre study of 119 advanced inoperable BCC patients treated with daily vismodegib, the objective response rate was 46% in locally advanced disease and 31% in metastatic BCC patients, respectively. Response was not associated with prior systemic therapy in patients with locally advanced BCC (p = 0.002) [20].
Conclusions
Vismodegib seems to be an effective and safe therapeutic approach also for radiation-related BCC, associated with relatively low toxicity. The pathways defined for the development of BCCs are interrupted tumour suppression pathways or altered activation of oncogenic signalling; moreover, UV and ionising radiation may induce mutations in pathogenic genes. Further studies are needed to understand the molecular biology for advanced BCC, the genetic basis of clinical response, and to activate strategies to evade acquired resistance.