Introduction
Endovascular aneurysm repair (EVAR) has become the preferred treatment modality for abdominal aortic aneurysm (AAA) [1, 2]. It is recognized that early and late outcome after endovascular procedures is comparable to and even better than open surgery to some extent [3–5]. There are some controversial arguments for the late survival benefit following the long-term results of randomized trials [3, 5–7]. The known early benefit of EVAR seems to be lost after several years [6, 7]. However, the OVER trial demonstrated non-consistent findings with the other 2 randomized trials [5]. Survival rates and identification of the predictors of late mortality after EVAR are important key factors to be assessed to ensure the real benefit of EVAR. Moreover, real world data along with randomized trials should be taken into consideration to make interpretations about outcomes and survival.
Aim
The aim of this paper is to emphasize the survival rates and predictors of reduced survival after EVAR procedure performed by a single team of endovascular surgery.
Material and methods
Patients
All patients who underwent endovascular AAA repair between January 2013 and December 2019 were identified retrospectively from the database of hospital records. Patient demographics, perioperative variables, and early and midterm outcomes were recorded from the hospital database and death certificates. This study follows the Declaration of Helsinki and the study design and protocol were reviewed and approved by the Institutional Review Board (Ankara Sehir Hastanesi, 1 Nolu Klinik Araştırmalar Etik Kurul Baskanliği, E1-19-161). The requirement for informed consent was waived due to the retrospective nature of the study.
Indications for EVAR included (1) AAA > 5.5 cm in maximum diameter, (2) saccular type of aneurysms, and (3) symptomatic aneurysms. Patients with hostile neck (defined as neck length ≤ 10 mm and/or reverse conical shaped necks) were all included in the study.
EVAR procedure
All EVAR procedures were performed in a hybrid operating room by a specific team of cardiovascular surgery. The unibody endografts were mainly used in the early period of the study. In more recent years, modular endografts have been used in our practice. The devices used in this study were the Ankura™ AAA (Lifetech) in 89 (40.1%) patients, the AFX® (Endologix) in 68 (30.6%) patients, the Endurant™II (Medtronic) in 58 (26.1%) patients, the Gore® Excluder® (Gore) in 5 (2.3%) patients, and the E-vita Abdominal XT (Jotec) in 2 (0.9%) patients.
The procedures were performed in the hybrid room under general (169 patients, 76.1%) or loco-regional anaesthesia (53 patients, 23.9%), based on the preference of the surgical team, anaesthesiologist, and patient. Modular endografts were deployed in standard fashion, and the technique for unibody endograft deployment has been previously described [8]. The Endologix AFX® device (unibody) consists of a main bifurcated unibody and a proximal aortic extension. This endograft is the only graft with anatomical fixation at the aortic bifurcation. The aortic extension is placed at the infrarenal position. Completion angiography was performed after the procedure. Type 1 endoleaks were treated by balloon angioplasty and placement of an extension cuff if needed. Type 2 endoleaks were followed.
Postoperative surveillance
Postoperative evaluation consisted of clinical and radiological assessment at discharge, 1 month, 6 months, 12 months, and annually thereafter. Computed tomographic examination and Doppler ultrasonography were performed at 1 month. If there were no type 1 or 3 endoleaks at first evaluation, subsequent assessments of endoleak and sac diameters were performed only by Doppler ultrasonography. Type 2 endoleaks were also assessed only by Doppler ultrasonography, because they are accepted as benign endoleaks in the absence of sac enlargement. If there was suspicion of sac enlargement at ultrasonographic examination, this finding was checked by tomography. Sac enlargement was defined as minimum of 5 mm enlargement compared to the preoperative diameter. Contrast angiography was performed at the hybrid room only when a secondary intervention was needed.
Estimating the possible predictors for survival
Possible predictors were assessed by 2 commonly used risk models for vascular surgery: the Vascular Quality Initiative and the Vascular Study Group of New England risk prediction models [9–11]. The combined possible predictors of these 2 risk models were analysed in the current study as follows. The predefined patient demographics (age as a continuous variable, gender, and comorbid factors such as diabetes, chronic obstructive pulmonary disease, and concomitant cardiac disease) were identified. Urgent repair was defined as symptomatic aneurysm treated within 24–48 hours of admission. Preoperative renal insufficiency was defined as creatinine level ≥ 1.8 mg/dl, and preoperative anaemia was defined as haematocrit values below 30%. Furthermore, aneurysm diameter was categorized as < 6.0 cm or ≥ 6.0 cm [12].
Statistical analysis
The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk test) to determine the normality of their distribution. Normally distributed continuous variables were expresses as mean ± standard deviation (SD) or median values with range if not normally distributed. Categorical variables were expressed as number and percentages. Demographic parameters, operating variables, and follow-up data were compared using the Mann-Whitney U test and χ2 test. Wilcoxon test was conducted to analyse pre-operative and follow-up diameter of aneurysm sac. Kaplan-Meier analysis was conducted to demonstrate freedom from all-cause mortality and freedom from secondary interventions. The hazard ratio (HR) and 95% confidence intervals (CI) were estimated with different Cox proportional hazard models to estimate the independent predictors of survival with adjustment of the predefined possible rick factors. A p-value of < 0.05 was considered to be statistically significant, and all statistical analyses were performed using the SPSS for Windows version 15.0 statistical software program (SPSS Inc., Chicago, IL, USA).
Results
A total of 222 patients underwent EVAR procedure in a 7-year period, and the procedures were carried out by the same team of cardiovascular surgeons. Baseline characteristics of the patients are summarized in Table I. The median age of the patients was 70 years (range: 46–92 years) and the study population was predominantly male (202 patients, 91%).
Table I
Baseline characteristics of the patients
The median stay in the intensive care unit and in the hospital was 4 hours (range: 2–240 hours) and 2 days (range: 1–20 days), respectively. Perioperative features are reviewed in Table II.
Table II
Perioperative features
In-hospital mortality was 1.8% (4 patients out of 222). Follow-up was available in all 218 survivors. Excluding 4 in-hospital mortalities, routine follow-up of remainders was included in the outcome assessment. The median follow-up period was 20 months (range: 1–80 months). The follow-up data are shown in Table III.
Table III
Follow-up data
Five-year freedom from any endoleak was 65% with a 95% confidence interval (CI) of 54.3–74.9%. On the other hand, freedom from type 1a endoleaks, type 1b endoleaks, type 2 endoleaks, and type 3 endoleaks was 97.5% (95% CI: 95.1–99.9%), 92.5% (95% CI: 85.2–99.8%), 83.9% (95% CI: 74.1–93.7%), and 86.5% (95% CI: 75.5–97.5%), respectively.
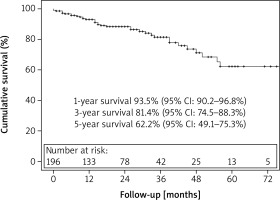
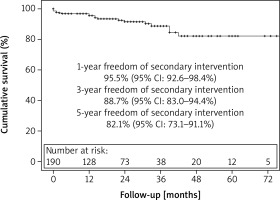
There were 36 (16.5%) late deaths. Kaplan-Meier survival analysis revealed that overall survival at 1, 3, and 5 years was 93%, 81%, and 62%, respectively (Figure 1). Freedom from secondary intervention was 96%, 89%, and 82% respectively for 1, 3, and 5 years (Figure 2). Indications for secondary intervention (endovascular or open) were type III endoleak (6 patients), stent-graft limb thrombosis (4 patients), type Ib endoleak (4 patients), type Ia endoleak (3 patients), and vascular access problems (2 patients) (Table III).
Aneurysm sac diameter tended to be decreased after the procedure regarding to the preoperative measurements (from median 60 mm to 58 mm, p = 0.047). At the follow-up period, 86% of aneurysms were detected to be decreased in size or remained stable, when considering an increase of 5 mm of diameter as an enlargement.
Predictors of survival
Multivariate Cox regression models revealed that the independent predictors for late mortality were the creatinine ≥ 1.8 mg/dl (adjusted HR = 2.68; 95% CI: 1.21–6.42; p = 0.027), hemoglobin < 10 g/dl (adjusted HR = 3.38; 95% CI: 1.16–9.90; p = 0.026), ejection fraction < 30% (adjusted HR = 5.67; 95% CI: 1.29–24.86) and AAA diameter ≥ 6.0 cm (adjusted HR = 2.20; 95% CI: 1.01–4.81; p = 0.049) (Table IV). Age, gender, and symptomatic status of the patient did not interfere with the late survival of the patients. For further analysis, follow-up variables such as endoleak presence, endoleak type, reintervention, and sac enlargement (> 5 mm) were also assessed by hazard models. Among these covariates, sac enlargement was found to be related with survival only in univariate analysis (HR = 3.78, 95% CI: 1.15–12.45; p = 0.029).
Table IV
Predictors of survival (unadjusted and adjusted hazard ratios analysed by Cox regression analysis)
| Parameter | Unadjusted analysis | Adjusted analysis* | ||||
|---|---|---|---|---|---|---|
| P-value | HR | 95% CI | P-value | HR | 95% CI | |
| Age (continuous variable) | 0.056 | 1.05 | 1.00–1.10 | |||
| Gender | 0.209 | 0.04 | 0.00–5.86 | |||
| Creatinine > 1.8 mg/dl | 0.002 | 3.70 | 1.61–8.48 | 0.027 | 2.68 | 1.21–6.42 |
| Haemoglobin < 10 g/dl | 0.003 | 4.91 | 1.71–14.13 | 0.026 | 3.38 | 1.16–9.90 |
| COPD | 0.716 | 1.14 | 0.57–2.25 | |||
| DM | 0.572 | 0.80 | 0.36–1.75 | |||
| CAD ± CABG | 0.107 | 1.78 | 0.88–3.57 | |||
| CABG only | 0.504 | 1.28 | 0.63–2.60 | |||
| EF < 30% | 0.023 | 5.44 | 1.26–23.56 | 0.021 | 5.67 | 1.29–24.86 |
| Symptomatic aneurysm | 0.494 | 1.27 | 0.64–2.53 | |||
| AAA diameter ≥ 6.0 cm | 0.010 | 2.70 | 1.27–5.75 | 0.049 | 2.20 | 1.01–4.81 |
| Follow-up variables: | ||||||
| EL | 0.944 | 0.97 | 0.40–2.34 | |||
| EL other than type II | 0.091 | 2.14 | 0.89–5.17 | |||
| Re-intervention | 0.485 | 1.40 | 0.54–3.62 | |||
| Sac enlargement (> 5 mm) | 0.029 | 3.78 | 1.15–12.45 | |||
Discussion
This report presents the mid-term outcome (median follow-up of 20 months) and predictors of survival after endovascular procedures with the use of all type of available endografts for the treatment of AAA by our single endovascular team. Freedom from all-cause mortality was 93% at 1 year, 81% at 3 years, and 62% at 5 years. The main predictors of lower survival rates after EVAR were poor ventricular function, aneurysms above 6 cm, and various comorbidities such as decreased renal function and anaemia.
In the past 2 decades, EVAR has increasingly become the standard treatment modality for non-complex infrarenal AAA [2]. EVAR has numerous advantages, especially in early survival benefit compared to open surgery, including the fact that the procedure has a minimally invasive nature and has a shorter recovery period.
During a median follow-up of 20 months 36 (16.5%) deaths occurred in our study population. The 5-year overall mortality was documented as 73.6% in a meta-analysis of 4 randomized trials [3]. The main controversy of this reported value is the selection criteria of the patients in the randomized trials, and all the patients in the trial were within the limits of the IFU. The numbers in the real world are slightly different from those in the randomized trials. The ENGAGE registry, which documented the outcomes of a single endograft (Medtronic Endurant™), reported that 17.8% of 1263 patients were outside the IFU limits and more than 10% had hostile necks. The 5-year overall survival rate was reported as 67% in the ENGAGE registry [13]. Other earlier real-world reports have shown similar survival rates between 63% and 72% as well [4, 12, 14–19]. The survival rates of the current study occurred as 93% at 1 year, 81% at 3 years, and 62% at 5 years, which is highly comparable with the real-world data. On the other hand, Jeon-Slaughter et al. demonstrated that inferior mid-term survival after EVAR is independently associated with larger AAA diameters, especially above 6.0 cm [12]. Five-year survival rates of < 6.0 cm and ≥ 6.1 cm were 73% and 52%, respectively, in the current study, which is comparable with the above-mentioned report.
Anatomical factors predicting survival after EVAR are the main topic of several studies in the literature [9, 10, 12, 20–22]. Aneurysm diameter, the anatomical properties of the aneurysm, and the neck angle were determined to be associated with midterm survival [22]. The initial aneurysm diameter independently predicted mortality in the long term. There was an almost 3-fold increase in mortality risk among patients with initial aneurysm diameter ≥ 6.0 cm in this study. Similarly, a recent study investigating the Vascular Quality Initiative database demonstrated a 1.5-fold increase of 5-year mortality at patients with large aneurysms (≥ 6.5 cm) [23]. Jeon-Slaughter et al. reported increased mid-term mortality risk with aneurysm size greater than 6.0 cm [12]. Furthermore, shorter life expectancy and higher rupture risk for endovascularly treated large aneurysms were documented by Zarins et al. [24]. The median diameter of AAA in our series was 60 mm, 58% of which were equal to or above 60 mm. The majority of reports and registries assessing predictors for mortality show a mean aneurysm diameter of between 5.5 and 6.0 cm [9, 10, 21, 24]. The registry of the Vascular Quality Initiative, which is composed of over 18,000 EVAR patients, reported to have aneurysms above 6.0 cm in only 24% of the registry [9]. Moreover, the mean aneurysm diameter was reported as 58 mm in the Vascular Study Group of New England risk prediction model [10]. The 3-fold increase of mortality risk in our series, which is more than other series, may be clarified by the relative increased diameter of our patient population.
In addition to aneurysm diameter, some demographic features and comorbidities have been found to decrease survival for EVAR patients [9, 11, 21]. The simplified risk score model that was mentioned in the report of Neal et al. recognized that low ejection fraction has a very high score (+5 score) for risk prediction [11]. Similarly, preoperative ejection fraction below 30 was predictive of mortality, with a 5-fold increased risk in our study. Piffaretti et al. reported a similar predictive value of heart failure on late all-cause mortality [25]. In addition, several other studies also have documented heart failure as a risk factor for long-term mortality [26–29].
On the other hand, age, gender, and some comorbidities such as diabetes and chronic obstructive pulmonary disease were not associated with survival. The association between survival and age along with gender has been previously reported by numerous studies [9, 10, 21]. The majority of the studies reporting predictors of survival have a mean age of over 70 years [20, 25, 28]. A limited number of studies could not associate age with survival [30, 31]. The relatively young population and small number of study subjects may be the reason for the lack of a relationship between age and survival.
Gender is generally reported as a covariate for survival [28, 32–34], although there are some controversies about its predictive value in other studies [19, 28, 35–37]. Women who undergo endovascular repair tend to be older than men in most of the studies and the older age may contribute to its predictive effect. However, the median age of women in our study was not significantly different from the men’s age. The results of our study are nevertheless reliable in high-volume reports [19, 28, 35–37]. The other confounding issue was the male predominance in our study (91%), which may impede the clarification of results regarding gender.
Alternatively, overall all-cause mortality in the midterm was significantly 3 times lower for the patients without renal disease or anaemia. Saratzis et al. concluded that impaired renal function was independently associated with an increase mortality following EVAR [30]. Similarly, Khashram et al. identified baseline renal impairment (creatinine > 1.7 mg/dl) as an important predictor of survival [28]. Additionally, there are several other studies reporting hazard ratios between 1.6 and 2.1, and confirming the results of our study [34, 38, 39]. On the other hand, concerning anaemia, a few single-centre observational studies have reported an association with reduced mid- and long-term survival [38, 40]. Another observational study regarding severe anaemia (< 10 gr/dl), which is similar to our definition, reported 2.6-fold increased risk of in-hospital mortality after EVAR [41]. Furthermore, our study is unique given that poor mid-term survival is associated preoperative severe anaemia (HR = 3.4). Anaemia may be assoicated with a diminished cardiac reserve and other comorbid conditions. The underlying condition may be addressed to overcome this condition.
This study includes some notable limitations. Firstly, these results were analysed retrospectively and evaluated from a single endovascular team, which may have caused a selection bias. The overall number of the study population was relatively low. Secondly, unfortunately, the cause of late deaths could not be identified for some patients. Therefore, analysis for aneurysm-related death and non-aneurysm-related death could not be constructed for this study. Lastly, considerably short follow-up time may be a limitation to assess the predictors of survival accurately. On the other hand, projection of 5 year survival of this patient cohort is comparable to the literature.
Conclusions
The outcomes of EVAR patients in the current study demonstrates safety and acceptable durability of the endografts in AAA patients at 5 years with a survival rate of over 60% and freedom from secondary intervention exceeding 80%. Large aneurysms, low ejection fraction, poor renal function, and anaemia are independent predictors of reduced survival after endovascular repair of the aneurysm. Longer-term follow-up is expected to be reported through 10 years.