Summary
According to our knowledge, pain and bleeding problems during and after cardiac electronic device implantation are the most important problems of this procedure. In our study, we tried to examine the effects of wide-awake local anesthesia (WALA) anesthesia, a new local anesthesia method, on bleeding, pain control and hematoma formation in cardiac device implantation procedures and to publish our results. In our study, we obtained positive results indicating that WALA anesthesia is a safe method for cardiac device implantation, and we believe that conducting studies with larger populations focusing on this subject would be beneficial.
Introduction
Cardiac implantable electronic devices (CIEDs), including pacemakers, implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices, have undergone rapid technological progress in the last decade [1]. A recently published trend analysis has revealed that the rate of CIED implantation, while experiencing a slight decrease during the global pandemic, remains notably high, at up to 400 patients per 100,000 population [2]. The expansion of therapeutic CIED indications and the insertion of these devices in higher-risk patients have been linked to an increased risk of major and minor procedural complications. Pocket hematoma rates after CIED insertion have been reported to be higher in a subset of patients who are at high risk of bleeding due to anticoagulant or antithrombotic medication [3] or who have advanced chronic systemic diseases [4], and it is reported that pocket hematoma increases the risk of device infection [5]. Despite the existence of multiple studies on various strategies, such as peri-procedural vacuum drainage systems, pro-hemostatic agents, compression devices, or pre-procedural interruption or bridging of anticoagulants, none of these techniques have sufficient evidence to justify their regular implementation in preventing pocket-related problems [3, 6].
Peri-procedural and post-procedural pain control in patients receiving CIED implantation has also been the subject of research [7, 8]. Subdermal co-injection of epinephrine as a local anesthetic has been a common procedure due to its vasoconstrictor effect on α-adrenergic receptors located in the skin. This approach serves the dual purpose of minimizing bleeding and enhancing the efficacy of local anesthesia. Wide-awake local anesthesia (WALA) with epinephrine has been demonstrated to be effective, particularly in the fields of orthopedics and hand surgery, as it obviates the requirement for patient sedation and even the use of a tourniquet to block the blood supply to the proximal extremity [9].
Aim
Although the first and only MAITRE Study from 2016 on the use of local epinephrine in CIED implantation reported discouraging results in terms of increasing pocket hematoma [10], we hypothesized that the WALA technique might be useful and secure, particularly in patients who received CIED implantation while on anticoagulant therapy.
Material and methods
During the period from March 2021 to July 2021, participants who met the criteria for cardiac device implantation, lead reimplantation, and battery replacement were recruited for this study. The study design employed a single-centered approach, double-blind methodology, and randomized controlled trial framework. The research was conducted at the Cardiology Clinic of the tertiary care state hospital, and the Ethics Committee of Ankara City Hospital, Turkey, granted approval for the study (IRB decision no: E1-21-1690). A total of 42 patients were enrolled in the study, all of whom satisfied the specified inclusion and exclusion criteria. The inclusion criteria for this study consisted of adult patients aged 18 to 85 years who had indications for CIED implantation and required lead or battery replacement. The exclusion criteria for this study covered three criteria: patient refusal to provide consent, the presence of allergies to local anesthetics and epinephrine, and the presence of bleeding diathesis. The study involved a retrospective evaluation of patient charts to analyze demographic and clinical factors such as age, gender, type of injury, underlying conditions, and comorbidities. The endpoints of the study were bleeding, intraprocedural and postprocedural pain, and the detection of a pocket hematoma by ultrasonography or physical examination.
The patients were randomly assigned to two groups using a computer-generated random table. Group 1, consisting of 21 patients, received local anesthesia of lidocaine and epinephrine, referred to as the WALA group, whereas Group 2, also consisting of 21 patients, served as the control group and received local anesthesia of lidocaine. Based on the findings, a healthcare professional (scrub nurse) provided a WALA solution to Group 1, whereas Group 2 received solely lidocaine. The primary operating physician responsible for carrying out the insertion procedure was blinded to the specific composition of the anesthetic solution.
As previously reported, the recommended mixture for WALA solution was 1% lidocaine mixed with 1 : 100,000 epinephrine and 1 ml of 8.4% sodium bicarbonate per 100 mg (10 ml) of lidocaine to buffer its acidic effect [11, 12]. To obtain 50 ml of WALA solution at these solubility levels, we used 0.5 mg/1 ml of epinephrine, 25 ml of lidocaine 2%, 5 ml of 8.4% sodium bicarbonate, and 19 ml of 0.9% isotonic sodium chloride.
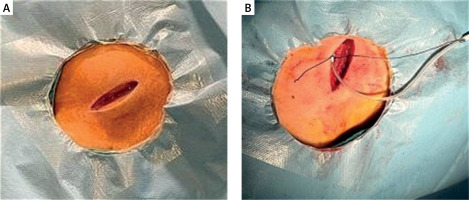
In the catheter laboratory, standard monitoring included pulse oximetry, electrocardiography, and noninvasive blood pressure monitoring. Standardized antiseptic measures were implemented for all patients. Cefazolin was administered intravenously 1 h prior to the procedure as prophylaxis for infective endocarditis. The procedure was conducted using local anesthesia in either the right or left subclavian region, without the use of moderate sedation. The operator had discretion in determining the pocket location, venous access, lead fixation type, and pacemaker mode. The preference was for subclavian access and subfascial pacemaker placement. Following the administration of a 20 ml WALA solution to the operative area, the operator observed a waiting period of 5–7 min. Rescue analgesia was administered based on the pain score assessed during the procedure. An additional dose of WALA solution exceeding 5 or 10 ml was administered. Electrocautery was employed for treating bleeding. Operative time was defined as the duration between the initiation of the skin incision and the completion of wound closure (Figures 1 A, B).
Figure 1
A – Image of subclavian access, B – image showing application of WALA solution to the operation area
After surgery, each patient was assigned a visual bleeding score from 1 to 10, with 1 indicating no bleeding and 10 indicating bleeding too severe to be managed without vein or artery ligation. Additionally, the duration of the surgery was documented. The Visual Analog Scale (VAS) was employed to assess pain and anxiety levels at 15-minute intervals during the entire surgical procedure, from the beginning of surgery until 24 h after the operation. Pain severity was categorized into three levels: mild (VAS 0–4), moderate (VAS 5–7), and severe (VAS 8–10). Breakthrough pain was defined as a VAS score of 4 or higher, either at rest or when requested by the patient. All patients were prescribed 6 h of bed rest and 3 h of compression therapy. Patients did not receive bridging anticoagulation. Surgery was performed on patients who were taking warfarin with an International Normalized Ratio (INR) between 2 and 2.5. Patients who were taking novel oral anticoagulants underwent surgery after a period of 24 to 36 h since their last dose. Antiplatelet medications, regardless of monotherapy or dual therapy, were not discontinued preoperatively and were administered as usual following surgery. Administration of hemostatic drugs was prohibited during the initial 2-day period following implantation. All patients underwent routine ultrasonography examination on the fifth day after implantation to identify pocket hematoma.
Statistical analysis
The statistical analysis utilized two software programs: Jamoviproject (2020), specifically Jamovi (Version 1.8.1), and JASP (Version 0.14.1.0). These programs were obtained from their respective websites: https://www.jamovi.org and https://jasp-stats.org. A significance level of 0.05 was used for all statistical analyses. The study was designed with the minimum number of participants due to lack of baseline knowledge about the effects of the WALA method on postoperative outcomes in patients receiving CIED. Descriptive statistics were provided for continuous variables, including the mean ± standard deviation and the median with minimum and maximum values, depending on the distribution of the variables. Categorical variables are presented as numerical values and percentages. The normal distribution of the numerical variables was assessed using the Shapiro-Wilk, Kolmogorov-Smirnov, and Anderson-Darling tests. The Mann-Whitney U test was used to compare two independent groups when the variables did not follow a normal distribution. The Pearson χ2 and Fisher’s exact tests were employed to assess differences in categorical variables between groups in 2x2 tables. Additionally, the Fisher-Freeman Halton test was used for comparing categorical variables in contingency tables.
Results
Table I provides a summary of the demographic and clinical characteristics of the patients. The median age of the patients was 67.5 years. Hypertension and coronary artery disease were prevalent comorbidities in 28 (66.7%) and 23 (54.8%) patients in the WALA and control groups, respectively. ACE inhibitors were the most common medications, used by 81% of the patients. There were no significant differences between the groups in terms of age, sex distribution, comorbidities, use of anticoagulant drugs, and medications (p > 0.05) (Table I). Table II provides the treatment details for the study groups. Heart failure was the predominant medical indication for implantation of CIEDs, with a prevalence of 52.4% in the WALA group and 71.4% in the control group. Regarding surgical indications, primary prevention was the predominant indication in 38.1% and 47.6% of patients in the WALA and control groups, respectively. Although the number of oral anticoagulant users was higher in the WALA group, there were no significant differences in the treatment details between the groups (p > 0.05), as shown in Table II. Table III presents the peri-procedural findings and complications. There were no significant differences between the groups in terms of postprocedural systolic and diastolic blood pressure, procedure duration, and the requirement for rescue analgesia (p > 0.05).
Table I
Demographic and clinical characteristics of patients
| Parameter | Overall (n = 42) | Groups | P-value | |
|---|---|---|---|---|
| Group 1 (n = 21) | Group 2 (n = 21) | |||
| Age [years]† | 67.5 [41.0–92.0] | 66.0 [47.0–92.0] | 69.0 [41.0–81.0] | 0.47* |
| Sex‡ | ||||
| Male | 26 | 12 | 14 | 0.23* |
| Female | 16 | 9 | 7 | |
| Comorbidities‡ | ||||
| Hypertension | 28 (66.7) | 16 (76.2) | 12 (57.1) | 0.33** |
| CAD | 23 (54.8) | 14 (66.7) | 9 (42.9) | 0.22** |
| DM | 13 (31.0) | 6 (28.6) | 7 (33.3) | 0.10** |
| Atrial fibrillation | 2 (4.8) | 2 (9.5) | 0 (0.0) | 0.49** |
| Use of anticoagulant drugs‡ | 4 (9.5) | 3 (14.3) | 1 (4.8) | 0.61** |
| Medications‡ | ||||
| ACE inhibitors | 34 (81.0) | 18 (85.7) | 16 (76.2) | 0.70** |
| B-blockers | 29 (69.0) | 13 (61.9) | 16 (76.2) | 0.50** |
| Mineralocorticoid receptor antagonists | 25 (59.5) | 12 (57.1) | 13 (61.9) | 0.10** |
| ASA | 24 (57.1) | 12 (57.1) | 12 (57.1) | 0.10** |
| P2Y12 receptor blockers | 15 (35.7) | 4 (19.0) | 11 (52.4) | 0.05** |
Table II
Comparison of treatment details in study groups
| Paramater | Groups | P-value | |
|---|---|---|---|
| Group 1 (n = 21) | Group 2 (n = 21) | ||
| Medical indications‡ | 0.40 | ||
| Heart failure | 11 (52.4) | 15 (71.4) | |
| AV complete block | 6 (28.6) | 5 (23.8) | |
| 2:1 AV block | 2 (9.5) | 0 (0.0) | |
| Slow AF | 1 (4.8) | 0 (0.0) | |
| VT arrest | 1 (4.8) | 0 (0.0) | |
| AV block | 0 (0.0) | 1 (4.8) | |
| Surgical indications‡ | 0.36 | ||
| CRT upgrade | 2 (9.5) | 0 (0.0) | |
| Implantation | 0 (0.0) | 1 (4.8) | |
| Replacement of lead and battery | 0 (0.0) | 1 (4.8) | |
| Pacemaker | 6 (28.6) | 3 (14.3) | |
| Primary protection | 8 (38.1) | 10 (47.6) | |
| Replacement | 4 (19.0) | 6 (28.6) | |
| Secondary protection | 1 (4.8) | 0 (0.0) | |
| Surgical procedure‡ | 0.31 | ||
| Implantation | 17 (81.0) | 13 (61.9) | |
| Replacement | 4 (19.0) | 8 (38.1) | |
| Device type‡ | 0.55 | ||
| CRT-D | 3 (14.3) | 5 (23.8) | |
| DDD PACE | 8 (38.1) | 5 (23.8) | |
| PACEMAKER | 0 (0.0) | 1 (4.8) | |
| Single Lead ICD | 9 (42.9) | 10 (47.6) | |
| Single Lead PACE | 1 (4.8) | 0 (0.0) | |
| Company‡ | 0.33 | ||
| Biotronik | 12 (57.1) | 15 (71.4) | |
| Boston | 9 (42.9) | 5 (23.8) | |
| St. Jude | 0 (0.0) | 1 (4.8) | |
Table III
Comparison of operative details and complications in study groups
| Parameter | Groups | P-value | |
|---|---|---|---|
| Group 1 (n = 21) | Group 2 (n = 21) | ||
| Post-procedural systolic BP [mm Hg]† | 135.0 [120.0–160.0] | 140.0 [115.0–160.0] | 0.21* |
| Post-procedural diastolic BP [mm Hg]† | 74.0 [35.0–90.0] | 78.0 [55.0–90.0] | 0.18* |
| Length of procedure [min]† | 40.0 [15.0–300.0] | 35.0 [20.0–90.0] | 0.67* |
| Intra-procedural pain score† | 2.0 [0.0–6.0] | 4.0 [2.0–8.0] | < 0.001* |
| Post-procedural pain score† | 1.0 [0.0–4.0] | 3.0 [1.0–7.0] | < 0.001* |
| Rescue analgesia† | 1 (4.8) | 0 (0.0) | 0.10** |
| Complications | |||
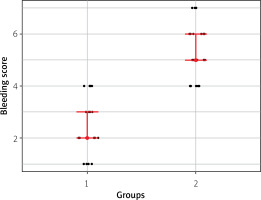
| Bleeding score† | 2.0 [1.0–4.0] | 5.0 [4.0–7.0] | < 0.001* |
| Pocket hematoma‡ | 1 (4.8) | 3 (14.3) | 0.61** |
| Pneumothorax‡ | 1 (4.8) | 0 (0.0) | 0.10** |
| Pocket and lead infection | – | – | – |
| Methemoglobinemia | – | – | – |
The control group had a significantly higher median bleeding score compared to the WALA group (5 vs. 2, p < 0.001) (Figure 2). The post-procedural pain score was significantly lower in the WALA Group compared to the control group (1 vs. 3, p < 0.001). Additionally, the control group exhibited a significantly higher median intraprocedural pain score compared to the WALA group (4 vs. 2, p < 0.001). The groups experienced similar complications. Although pocket hematoma was more common in the control group, there was no statistically significant difference between the two groups. No surgical revision was necessary for any of the patients with pocket hematoma, and their symptoms improved with conservative treatment. No cases of CIED infection occurred in either group.
Discussion
Considering the lower pain scores reported by patients both during and after the procedure, it is obvious that WALA not only provides superior local anesthesia during surgery but also maintains its analgesic effect for a period of time after the surgery. None of the patients experienced epinephrine-induced hemodynamic effects, and staying awake they provided more accurate pain assessments, resulting in more effective analgesia with additional dose injections when required.
Although serious complications such as cardiac arrest, acute coronary artery syndrome, cardiac perforation, pericardial tamponade, or hemothorax have been reported during CIED implantation, the overall risk of these complications does not exceed 3 to 8%. Pocket hematoma and secondary implant infection have also been defined as significant complications with reported occurrence rates of up to 9%. These complications have important long-term consequences and deserve particular attention in terms of prevention [13]. In our study, the significantly lower bleeding scores observed in the WALA group suggest that the technique may be advantageous for managing bleeding during the procedure. The MAITRE study [10], the only study on the topic, examined the effects of adding epinephrine to the local anesthetic solution during CIED implantation on pocket hematoma and the necessity of drain placement. According to the study, the addition of local epinephrine to the local anesthesia solution had no adverse hemodynamic effects; however, it was the only independent factor that significantly increased the risk of pocket hematoma (OR = 5.95, 95% CI: 2.1–7.3, p = 0.003). The authors attributed this to the local vasoconstrictive effect of epinephrine, which temporarily stopped punctate hemorrhages and led surgeons to avoid using drains. The authors also mentioned cases in which epinephrine caused epidermal necrosis due to vasoconstriction of the skin.
In our study, the observed disparity in pocket hematoma rates between groups was not statistically significant due to the limited number of affected patients. The high incidence of pocket hematoma in the control group may be attributable to the fact that nearly half of the patients received dual-antiplatelet therapy preoperatively without interruption. In contrast to the MAITRE study’s [10] results, the WALA group did not experience any instances of skin necrosis, and the pocket hematoma was only observed in 1 patient. In our view, an optimal level of anesthesia can be attained by adhering to the established quantitative values for the WALA solution. Specifically, it is important to ensure that the solution does not exceed the appropriate dosage of epinephrine and that the recommended dose of bicarbonate is used to neutralize the acidic effects of lidocaine.
Expansion of CIED indications created an increase in cumulative experience, and advances in medical therapy for coronary artery disease and heart failure have resulted in an increased quality-of-life expectancy in patients who have survived longer compared to the past [14]. Furthermore, the field of cardiac electrophysiology has exhibited a progressive divergence from cardiology over the past decade, as evidenced by the increasing disparity between the two subspecialties [2]. These advancements influenced clinical practice to develop criteria for high-quality CIED implantation: surgical dexterity for a bleeding-free procedure, avoidance of intravenous sedation to achieve rapid recovery, and negligible pain without the need for opioids. However, approximately one-third of patients still require systemic analgesics and sedation prior to and during the procedure [15], and the European consensus advises prompt surgical revision in case of unbearable pain due to hematoma formation, despite the potential risk of pocket infection [16]. The effectiveness of the WALA solution for peri- and post-procedural pain control during surgery lies in the addition of sodium bicarbonate and epinephrine to lidocaine. This combination reduces the painful effects of lidocaine, which are caused by its acidic pH. As a result, the use of the WALA solution leads to reduced pain at the beginning of the operation, as well as during and after the procedure [11]. In our opinion, using the WALA technique will be an effective and safe way to avoid potential problems with CIED implantation and to meet modern quality standards for all types of cardiac electrophysiology procedures, which are likely to be performed more frequently in the future.
Our study had various limitations. The study was conducted at a single center with only two cardiologists performing the procedures. Neither operator had equal numbers of patients in the WALA and control groups. It is well established that bleeding complications can differ based on the operator’s level of experience. Additionally, this study is constrained by its limited sample size and brief duration of follow-up. The study’s small sample size is primarily intended to ensure the safety of the new technique. This study primarily investigated the anesthesia technique, its safety, and the intraoperative experience of both the patient and surgeon. Third, the study’s primary outcome measures were subjective in nature, relying on pain scores reported by patients and bleeding scores assessed by operators.
Conclusions
WALA anesthesia significantly reduces intra- and post-procedural bleeding and pain. The potential benefits of the technique, such as preventing the formation of pocket hematoma, shortening the duration of the procedure, and accelerating the recovery period, must be demonstrated in randomized trials conducted on a larger number of patients.