WIST Guilelines are published simultaneously in “Cardiovascular Revascularization Medicine”.
Summary
The World Federation for Interventional Stroke Treatment (WIST) establishes an individualized approach to acquiring clinical knowledge and procedural skills to meet the competency requirements for certification of interventionalists of various disciplines and stroke centres in endovascular treatment (EVT). WIST guidelines encourage acquisition of skills using innovative training methods such as structured supervised high-fidelity simulation and procedural performance on human perfused cadaveric models. WIST multispecialty guidelines outline competency and quality standards for physicians and centers to perform safe and effective EVT. The role of quality control and quality assurance is highlighted.
The World Federation for Interventional Stroke Treatment (WIST)
The WIST is a non-profit organization established in 2015 by multispecialty experts in interventional stroke treatment. Specialties involved in the WIST include neuroradiology, radiology, neurosurgery, cardiology, anaesthesiology, neurology, stroke, and vascular medicine. The aim was and remains to increase awareness of the importance of interventional stroke treatment and provide structured, specific training in acute stroke intervention. In addition, WIST serves as a forum for information exchange in the field of acute ischaemic stroke intervention with special emphasis on a multidisciplinary collaboration. The WIST offers continued, practical, case-based education, which includes multidisciplinary team training, remote and on-site proctoring, and advice on patient management. In addition to the annual ICCA-Stroke (Acute Stroke Interventions and Carotid Stenting) meeting, site-specific educational events are held on a regular basis. The training portfolio includes dedicated simulator and cadaveric training in cerebral angiography, acute stroke intervention, and carotid stenting as well as recognition and management of complications. Furthermore, the WIST assists in setting up thrombectomy services with standard operating procedures, and in designing institutional and inter-institutional patient diagnosis and treatment pathways. The WIST provides operator and centre certification and continued audit. The WIST is a multispecialty organization that is by design independent of vested interests. It has a track record of assisting effective establishment of Mechanical Thrombectomy (MT) centres across the world. Based on the collective experience of experts from multiple specialties involved in endovascular stroke treatment, the WIST has a unique position to outline training guidelines for acute stroke intervention.
Introduction
Ischaemic stroke is the leading cause of disability worldwide and a leading cause of death [1]. Long-term disability and/or death is most often caused by a large cerebral vessel occlusion (LVO). The focus is to re-establish cerebral blood flow as soon as possible. While the clinical benefit of intravenous thrombolytic therapy is widely accepted [2–6], its efficacy is limited. Only a small proportion of patients are eligible for intravenous thrombolysis due to late presentation and contraindications. Intracranial haemorrhage is a relatively common complication after systemic thrombolytic therapy, causing neurologic status deterioration in ~6–9% of patients [2–6]. Importantly, the neurologic outcome correlates closely with recanalization rates [7], but in patients with internal carotid, tandem or T-occlusion, or occlusion of the middle cerebral artery (MCA), a minority (~4–20% depending on the vascular distribution) will have a patent vessel after intravenous thrombolytic therapy [7, 8]. In fact, proximal location (e.g. internal carotid artery/MCA origin) and thrombus volume [9] of an occluded vessel are independently associated with poor recanalization rates [10]. Finally, the likelihood of death or major disability remains high (> 50%) [2, 6, 11]. Over the last 2 decades tremendous efforts have concentrated on improving vessel recanalization rates. It is not likely, however, that any further gain with systemic thrombolytic therapy can be achieved in the rate of cerebral vessel recanalization without increasing the haemorrhage rate.
Intra-arterial thrombolysis
Although intra-arterial thrombolysis may improve angiographic results after failed or incomplete endovascular treatment (EVT) [12], the role of stand-alone intra-arterial thrombolysis, as compared to IV-thrombolysis and its dose in patients with distal emboli is not established, and further research is needed.
Similarities and differences to primary percutaneous coronary intervention (PCI)
The approach to the treatment of ischaemic stroke resembles that of myocardial infarction. Both are medical emergencies caused by acute vessel occlusions. Mechanical myocardial recanalization demonstrated better myocardial muscle salvage [13], higher vessel patency [14], and significantly better clinical outcomes with reduction in mortality and intracranial haemorrhages [15]. Stroke therapy has undergone an almost identical evolution, from systemic thrombolytic therapy associated with limited vessel recanalization and risk of intracranial haemorrhage, to reperfusion strategies based on endovascular clot extraction. However, in general, the critical tissue window in stroke is markedly narrower than in acute myocardial infarction. Hence, cerebral endovascular stroke treatment should be viewed as cerebral resuscitation.
Endovascular stroke treatment
Several randomized trials have demonstrated the tremendous benefit of endovascular stroke treatment, with reduction of patient disability and mortality [16–19]. The approved time window for thrombectomy is longer than for thrombolysis. Furthermore, it has been demonstrated that the time window for MT can be extended when neuroimaging demonstrates a significant mismatch between infarct core and viable brain tissue (i.e. patients with a large penumbra) [20, 21]. In anterior circulation LVO it has been shown that, when MT combined with systemic thrombolysis is compared with IV-thrombolysis alone, the number needed to treat (NNT) to improve neurological outcome (by one level on the modified Rankin Scale [mRS]) is only ~2–3 patients [22]. The trials provide Class 1, Level A evidence that endovascular thrombectomy should be the standard of care for patients with acute cerebrovascular occlusions, and it is now considered the treatment of choice [23, 24].
However, training in endovascular stroke treatment is limited. This is related to: a) the small number of specialists currently trained to perform this procedure, b) the insufficient number of centres able to deliver EVT in many healthcare systems, and c) suboptimal geographical distribution of centres that negatively impact patient transportation time.
The epidemiology of acute ischaemic stroke (AIS) is complex. Using literature-based projections of LVO cerebral ischaemia patients treatable within 8 h of onset, endovascular treatment would be suitable for 21.4% of AIS patients. Importantly, these events account for 34% of post-stroke dependence and death and 52.8% of post-stroke mortality [25]. Although LVOs only cause approximately one-third of AIS, they are responsible for three-fifths of dependency and more than nine-tenths of mortality after AIS [25].
However, based on a recent survey of European countries, only a minority of patients with ischaemic stroke who would be eligible for EVT received it [26]. Consequently, many are left with life-altering disabilities that may have been avoided, had the therapy been available locally.
Importance of time
Time to treatment is at least equally as important and may be more important in AIS patients compared to those with acute myocardial infarctions. For example, if cerebral infarct vessel recanalization can be established within 180 min of symptom onset, a favourable neurological recovery rate (mRS of 0–2) is achieved in > 60%, whereas it is only ~46% if recanalization occurs 480 min after symptoms onset [27]. Furthermore, for every 9 min delay from symptom onset to reperfusion, one of every 100 patients will have a poorer disability outcome (i.e. higher mRS) [27]. Thus, a 1–2 h time delay (e.g. for air or ground transport) is undesirable. The aim must be to provide swift and safe acute stroke care, including endovascular stroke treatment, to every patient – regardless of their postcode and location, recognizing that any delay causes preventable disability to many patients. To reach this goal as quickly as possible, it requires training of an adequate number of interventionalists to offer the benefit demonstrated in randomized trials without jeopardizing safety. The assumption that area-wide stroke coverage, including remote geographical locations, can be provided by one specialty is unrealistic.
WIST-training concept
Structured training without barriers between specialties is essential. It has long been demonstrated that quality interventional stroke care can be delivered by different specialists such as neuroradiologists, cardiologists, radiologist, neurosurgeons, vascular specialties, or neurologists experienced in endovascular procedures [28–33]. This highlights the importance of good training guidelines.
This Training Guideline provides requirements for the theoretical and practical training needed to become a stroke interventionalist who can safely offer mechanical thrombectomy and other endovascular procedures for AIS. After documented completion of the recommended training, the WIST can certify interventionalists and centres that are deemed competent to perform acute stroke interventions. As a progression to other guidelines and recommendations, special emphasis rests on 3D printed bench-model, simulator, team, cadaveric, and animal training. While the number of procedures recommended by other expert panels and societies [34–37] is reasonable and has been adopted in this statement, and the necessary training requirements can be completed in vivo during supervised procedures, our position statement deviates from prior recommendations and guidelines in the following 2 aspects:
First, the widely suggested 25 EVT cases can be performed via strictly supervised simulator/cadaveric training. The reason is that modern simulators, together with experienced instructors, offer cognitive and technical training that is equivalent and perhaps superior to, and safer than, hands-on in vivo on the patient training.
Second, candidates for WIST certification must demonstrate sufficient clinical and procedural knowledge by passing 2 standardized exams. The reason is that participation in courses or conferences alone does not necessarily translate into acquisition of the knowledge necessary to safely engage in MT. We stipulate recanalization and intracranial haemorrhage rates as previously suggested [34] and emphasize the importance of demonstrating and documenting service times and outcomes.
There are two pillars of training: clinical and procedural (Figure 1). The purpose of the clinical pillar is to support the stroke clinician in selecting patients in whom the benefit of EVT outweighs the risk. The second pillar is the acquisition of the skills necessary to perform endovascular stroke treatment, including recognition and management of complications.
Figure 1
WIST qualification and certification pathway. The described WIST qualification and certification pathway does not exclude other routes
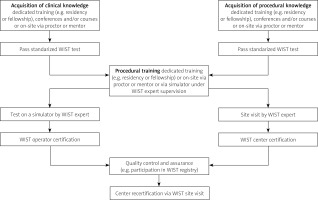
Clinical training requirements
The decision of whether to proceed with EVT optimally should involve both a stroke specialist and an interventionalist, regardless of whether the patient presents as a referral or is an “in house” patient. Whilst it would not be expected for the interventionalist to replace the clinical expertise of the stroke specialist or the imaging expertise of the reporting radiologist, the operator must, based on the clinical, laboratory, and neuroimaging information, be able to identify patients who meet the criteria for EVT. The interventionalist must be familiar with currently used stroke severity and imaging scales, and the vascular anatomy, and have the knowledge to recognize major complications during or after the procedure on clinical and neuroimaging grounds. This training may occur during courses or on-site within a hospital.
To be considered proficient, the trainee must have knowledge in the following areas:
Stroke treatment strategies.
The local stroke pathway.
Clinical assessment scores such as mRS, NIHSS.
Imaging modalities and their advantages and limitations.
Vessel anatomy and functional regions.
Endovascular devices.
The factors impacting patient selection including imaging information (infarct volume, penumbra, collaterals, vessel location, eloquent areas), clinical presentation, blood pressure, and underlying diseases.
Pre-, peri-, and postprocedural management.
To demonstrate proficiency in the aforementioned clinical areas, the trainee must pass a standardized test administered and certified by the WIST.
Procedural training requirements
Given the familiarity with the basics of vascular access and navigation of large and small arteries by practitioners performing endovascular procedures, the focus of training is specifically to establish access to and navigate the cerebral vasculature with wires, catheters, and devices used to perform endovascular stroke treatment and how to avoid, identify, and, if needed, manage procedural complications.
The trainee must demonstrate knowledge and skills in the following areas:
Arterial and venous anatomy with emphasis on cerebral vessels that require navigation during stroke interventions including common variants and cerebral territories and their neurological function supplied by the respective vessels treated by endovascular means.
Identification of a difficult arch and how to navigate it.
Establishment of carotid and vertebral artery access.
Carotid artery thrombectomy and stenting including familiarity with proximal and distal protection, stent designs, balloon angioplasty, thrombectomy catheters, and complication recognition and management [38].
Wiring tortuous cerebral anatomy.
Preparation and use of equipment for EVT such as balloon tipped sheaths, cerebral microcatheters, thrombectomy devices, stent retrievers, and aspiration catheters.
Catheter-based local intra-arterial lytic therapy.
Management strategies for persistent vessel occlusion after first- or multiple-pass attempts of thrombectomy devices.
Recognition and management of procedural complications including but not limited to distal embolization, thrombus migration, vessel dissection, and perforation.
Antithrombotic and antiplatelet medications.
Patient and arterial blood pressure management.
Pros and cons of local versus general anaesthesia in different scenarios.
Demonstration of sufficient knowledge of the above areas requires passing a written standardized test administered by the WIST.
Hands-on practical training
Before thrombectomy is independently performed by an interventionalist, in addition to passing the previously mentioned standardized clinical and procedural knowledge tests, a minimum number of cerebrovascular interventions is required. These may have already been performed by the applying physician during their clinical practice or can be acquired during supervised training on high-fidelity simulators or human cadaveric models.
The above-described procedures can be performed at any institution as primary operator or under the guidance of a proctor (documented in a procedure log) and/or under the guidance of a WIST qualified proctor using a high-fidelity simulator meeting predefined metrics. This will cover a range of scenarios including emergency and rescue procedures.
Proficiency must be demonstrated by simulated acute stroke interventions at a WIST qualified simulation centre.
A successful acute stroke/MT program is the result of a team effort, which ideally includes a stroke and imaging specialist and an interventionalist able to perform endovascular stroke treatment and support staff with sufficient knowledge of pre-, peri-, and postprocedural stroke care and able to manage arterial line and invasively monitored blood pressure. The WIST certifies, on an annual basis, interventional centres that are deemed competent to perform acute stroke interventions.
Centre certification requirements
Comprehensive training undertaken by all key specialties of the thrombectomy service according to the guidance of the WIST.
Evaluation of the stroke/thrombectomy service during a WIST inspection visit.
Key elements that must be identified during the inspection visit are as follows:
Required hospital environment:
Engagement of hospital leadership and specialties involved in stroke care pathways.
Governance lead for the endovascular stroke service.
Governance for pathways selection, imaging, pre-, peri- and post-procedural care in place.
Named lead for Hospital Quality Management that guides the process.
Storage of patient and procedure critical data to ensure data availability for internal and external audit including symptom and door to arterial puncture, device deployment, and recanalization time.
Conduct of internal audits with integrated quality improvement process. The goal of this is to optimize the pre-, peri-, and post-procedural processes and thus improve patient outcomes.
Availability of the necessary equipment and staff to plan and perform endovascular stroke procedures:
The interventional department is equipped with the necessary infrastructure; staff is trained in modern imaging techniques and able to provide support to the service.
The interventional suite is suitable to perform endovascular stroke treatment with or without general anaesthesia.
Dedicated equipment and devices are available.
The intensive care unit is prepared to take over thrombectomy patients, when necessary.
There is access to a stroke unit, monitored beds, and rehabilitation services.
Presence of a trained team able to support and perform the endovascular stroke service and define patient care plans:
Clear communication between all team members dedicated to providing a thrombectomy service of excellence.
Clear distribution of responsibilities within the team.
The EVT team has received training in all aspects of the procedure and pathway.
Provision of peri-and post-procedural support is ensured.
The interventional team is experienced in intra-arterial procedures and demonstrate proficiency in thrombectomy and related procedures.
Interventional staff is capable to deal with standard and emergency procedures.
Interventional staff have been trained in procedural steps.
World Federation for Interventional Stroke Treatment (WIST) Center Re-certification
Annual re-certification is granted by the WIST on the basis of data submission for external auditing, including times and outcomes. Re-certification typically includes an inspection visit by a WIST auditor.
Maintenance of Physician Qualifications
It is vital that physicians have ongoing, stroke specific, continuing medical education. A minimum of 16 h of stroke-specific education every 2 years is required. Individual physician outcomes should conform to institutional requirements, and national and international standards.
The quality improvement program aims to review data of all patients who receive interventional therapy for acute stroke.
Participation in the WIST registry is expected. Outcomes must be tracked and recorded. While threshold levels for recanalization, complication rates, etc. have yet to be established, we suggest the following as a minimum:
– Successful recanalization (defined as modified TICI 2b or 3) in at least 60% of cases.
– Less than 15% embolization to new cerebrovascular territory.
– Less than 10% intracranial haemorrhage on imaging with clinical deterioration [34].
The WIST recommends contribution of all centres to national audit databases so that progress and logistic issues and geographical equality for patients can be monitored and improved upon.
Hospital requirements
Successful treatment of acute stroke occurs with the framework of a multi-disciplinary team. It is critical that patients should be treated in a centre with access to the following:
Neurosurgery does not need to be available on site; however, for patients with cerebral bleeding or in need for decompressive surgery, cooperation with neurosurgery is required. A plan for prompt patient transfer to a centre able to provide neurosurgical support should be in place if this is not available at the respective institutions.
Summary
The WIST recommends dedicated thrombectomy training and quality assurance/improvement processes to ensure the best possible patient outcomes. Well-trained interventionalists are a critical component of an organized and efficient team needed to deliver clinically effective endovascular treatment for AIS patients.
We recognize that the specific training pathways may differ across geographic regions and health care systems, but the consensus is to mandate adequate training to perform emergent endovascular stroke intervention. These cognitive requirements consist of baseline training and qualifications as well as ongoing professional education, which are essential for safe and efficient patient management.
It is also important to point out that these qualifications are for new practitioners who are not currently performing acute stroke intervention with MT. We understand that there are current practitioners who may have trained prior to the establishment of formal training pathways and have acquired the necessary skills listed above to treat acute stroke patients safely and effectively.








