Purpose
Dermatofibrosarcoma protuberans (DFSP) is a locally aggressive rare soft tissue tumor, and traditionally has been associated with a high-rate of recurrence after surgical excision. It is a slow-growing, insidious tumor that may be misdiagnosed clinically, often delaying definitive diagnosis for many years after original presentation [1]. Data of the surveillance, epidemiology, and end results (SEER) program managed by the National Cancer Institute, reports the incidence of DFSP at 4.2 cases per million, accounting for approx. 0.1% of all cancers [2].
The primary treatment modality include surgical wide local excision, leading to complete resection of tumor with 1-3 cm margins (depending on anatomic location and approximation to critical structures). The local recurrence rate after surgery ranges from 0 to 63%, depending on the nature of surgery and ability to obtain widely negative margins [3-5]. In general, when final margins are negative, local recurrence is quite low and ranges from 0 to 13% [4-8]. However, with close or positive margins, local recurrence ranges from 21% to 82%, according to published series [4-6, 9].
The high-risk of local recurrence after inadequate surgical resection may be a reason to consider adjuvant radiotherapy when re-resection is not an option. However, due to rarity of DFSP, there are no prospective randomized studies evaluating the role of adjuvant radiation therapy [10]. Dermatofibrosarcoma protuberans is radio-sensitive, and adjuvant radiation therapy is associated with lower local recurrence rates, especially in cases with a positive margin, incomplete resection, or in inoperable tumors [11]. The current case highlighted the ease and advantages of using surface mould high-dose-rate brachytherapy (HDR-BT) in the treatment of scalp tumors. It adds to the limited evidence available on the use of this modality in sarcomas, especially in DFSP.
Case presentation
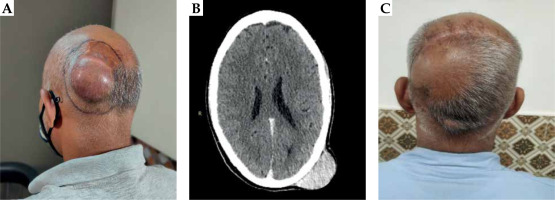
A 45-year-old men presented with complaints of swelling over left parietal aspect of the scalp, who underwent excision at a peripheral hospital. Post-operative histopathological examination report (post-op HPER) suggested a low-grade spindle cell tumor. Five years later, he developed a slowly growing local recurrence, which was excised after six months at a peripheral hospital. Post-op HPER revealed a low-grade spindle cell neoplasm. Six months after the second surgery, he suffered a third local recurrence and was referred to our hospital. At presentation in our hospital, a clinical examination showed a 6 × 6 cm well-defined, soft to firm, non-tender swelling present over left parieto-occipital region (Figure 1A). Core biopsy was suggestive of DFSP. Contrast-enhanced computed tomography (CECT) of the head and neck showed a well-defined hyperdense lesion in the scalp (size, 2.7 × 4.5 × 5.1 cm3) in the left parieto-occipital region, with no evidence of cervical lymphadenopathy or bone involvement (Figure 1B). The patient underwent a wide local excision with bilateral rotational advancement flap (Figure 2A). Post-op HPER showed neoplastic spindle cells arranged in a storiform pattern with moderate mitotic activity. Resection margins were free of tumor cells, with the closest margin (medial) being 0.4 cm away. Immuno-histochemistry (IHC) was positive for CD34, and negative for S100 and SMA, with a Ki-67 index of 30-40% in the most proliferative areas. The final diagnosis was of a DFSP. In the view of multiple recurrences, aggressive pathological features and technical difficulties anticipated with re-resection as well as adequate reconstruction that would be required in the scenario of a further recurrence, the patient was planned for adjuvant HDR-BT using a customized surface mould.
Fig. 1
A) Pre-operative image showing a 6 × 6 cm swelling over the left parieto-occipital region of the scalp. B) CT scan of the skull showing a hyperdense lesion in the scalp of size 2.7 × 4.5 × 5.1 cm3 in the left parieto-occipital region, with no evidence of bone involvement. C) Post-operative image showing well-healed scar of translocation flap
Treatment planning and delivery
A thermoplastic mask (three clamp head and neck) was applied for immobilization of the patient in a prone position.
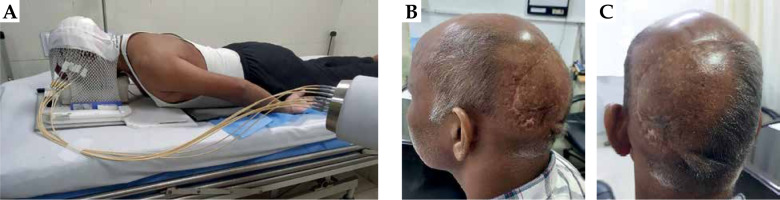
The tumor bed was then identified and drawn over the mask based upon initial clinical findings, pre-op CT images, and consultation with a surgical oncologist. A 2 cm margin was added to the tumor bed to mark target area (TA). The skin and subcutaneous tissue up to the skull bone of the scalp formed the depth of target volume. This depth was confirmed on CT scan to be at 5 mm from the skin surface. Layers of dental wax of 5 mm thickness were then applied and molded to the scalp curvature, with gentle pressure and simultaneous application of heat using a blow dryer. 07 parallel catheters were fixed at 1.2 cm distance followed by additional layers of dental wax, ensuring the stability and patency of catheters (Figure 2A). A separation of 1.2 cm was maintained to achieve a relatively homogenous dose distribution within the target volume at a depth of 5 mm.
Fig. 2
A) Patient in prone treatment position with thermoplastic mould and treatment catheters connected to HDR-BT machine. B, C) Photographs of the patient, 18 months after completion of brachytherapy. There is no evidence of recurrence or any radiation toxicities, except for mild hypopigmentation of the irradiated skin
CT scan (Brilliance CT-16® slice with Accusim virtual simulation; Philips Health Care Solutions, DA Best, The Netherlands) was performed using thin slice width of 2 mm from the vertex to the brow level (120 kv and 250 mAs). After CT simulation, the data was transferred to treatment planning system (Oncentra Brachy version 4.6.0, Nucletron, The Netherlands; MicroSelectron Brachytherapy system Model version 3 by Nucletron, The Netherlands).
Following image reconstruction, planning target volume (PTV) corresponding to the TA was contoured on axial slices, while reviewing the same on sagittal and coronal images. The PTV, consisted of the TA and a small additional lateral margin for positional and setup errors, was delineated according to wire markers visible on CT scans. The final implant size covering the PTV was measured 8 × 9 cm2. As mentioned earlier, the depth of target volume was 5 mm from the skin surface.
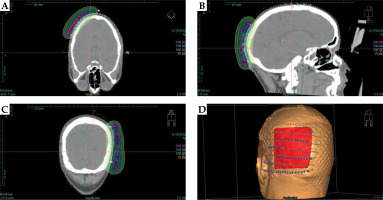
After contouring the TA, the skull bone and mould dose points were created around the catheters, and the dose was prescribed. Following this, volumetric optimization was performed to confirm the 100% prescription isodose corresponded to the target volume. A dwell position spacing of 5 mm was applied to ensure maximum dose homogeneity within the target volume. Care was taken to reduce the volume of the target covered by 150% and 200% isodose curves as far as possible (Figure 3A-D). Volume receiving 100% (V100) and 150% (V150) of the dose were calculated as 29.2 cc and 0.23 cc, respectively.
Fig. 3
A-C) CT planning images showing axial, sagittal, and coronal views, respectively, of the treatment plan with dose wash and isodose lines of 200% (magenta), 150% (light blue), 100% (green), and 75% (yellow) of the prescribed dose. D) Digitally reconstructed image with treatment catheters and dwell points around the target area (red)
The total prescribed dose was 42 Gy to the 100% isodose, with equivalent dose in 2 Gy (EQD2) of 47.25 Gy10, delivered in twice daily fractions of 3.5 Gy each, at least 6 hours apart for 12 fractions.
The radiobiological dose was a little less than but close to the recommended 50 Gy in 25 fractions (EQD2: 50 Gy10) for adjuvant EBRT in adjuvant setting [10]. Considering that the tumor bed would receive a dose higher than 100% prescription dose, the dosage was deemed radiobiologically adequate.
Due to logistic reasons, the patient received only one fraction on the first day, followed by 2 factions each on the next five days, and a single last fraction on the seventh day. The treatment was delivered during seven consecutive days, irrespective of the weekend or other holidays. The patient completed the treatment without any adverse effects, except for grade 1 skin reactions over irradiated area, which appeared at week 1 and healed within 4 weeks post-treatment. Eighteen months after treatment completion, the patient is on regular 3 months follow-up. At the most recent follow-up visit, the patient was asymptomatic and disease-free on clinical examination and CT scan of the head. No late toxicities were evident, except for a mottled hypopigmentation of the irradiated skin (Figure 2B, C).
Discussion
The standard of care for DFSP is surgical excision with a complete histologic margin examination [12]. As with other sarcomas, this type requires a substantial margin, which may generally be acceptable for tumors involving the trunk and extremities, but may induce significant cosmetic and functional morbidities in tumors involving the head and neck region. The use of adjuvant external beam radiotherapy (EBRT) or brachytherapy (BT) to enhance local control (LC) in patients undergoing limb-sparing sarcoma resections in the extremity is supported by level 1 evidence from randomized prospective clinical trials [13, 14]. Radiation therapy may be administered as pre-operative external beam therapy, or post-operatively as either EBRT or BT. However, there are no randomized data or consensus on whether it is preferable to use EBRT alone, BT monotherapy, or BT as a boost in various clinical settings, and decisions are taken at the discretion of treating physicians [15]. In the present case, RT was considered despite adequate surgical margins as an adjuvant therapy in view of multiple recurrences and high proliferative index (Ki67 being 30-40%) of the tumor. The case was discussed jointly in a multidisciplinary tumor board, and the surgical oncologist opined that re-resection in case of another recurrence would not be a feasible option due to currently available limited reconstructive options.
Our decision to use surface mould BT in this patient was influenced primarily by tumor location, resection at the time of next recurrence not being a feasible option, and no involvement of regional lymph nodes. Photon and electron EBRT plans were also considered as options. It was decided that due to the anatomic curvature of the region, there was a risk with photons of high exit dose, with unavoidable toxicity to the brain even after application of a bolus. Similarly, because of the curvature, the use of electrons with adequate margins would require the use of multiple beams to ensure adequate dose homogeneity. This would cause an increased difficulty and uncertainty in dose delivery, especially at junction areas during field matching. Thin skin and subcutaneous tissue in the scalp region also indicated that the use of electrons would lead to a high-dose deposition to underlying bone. Customized surface moulds ensured that HDR-BT catheters were uniformly placed in close proximity to the target area, even over the curved surface. The sharp dose fall-off of HDR-BT confirmed sparing of the underlying bone and brain. The treatment plans were deliberated upon, and a consensus was achieved to treat with surface mould HDR-BT, providing the highest conformity and appropriate treatment.
In a number of published articles, we found priority of the use of surface mould HDR-BT for treatment of superficial sarcomas, including the scalp [16-20]. Mitra et al. [16] described their use of surface mould HDR-BT in the treatment of 20 cases of angiosarcoma over 15 years, 75% of which had tumors in the scalp region. Indications were for both upfront and recurrent tumors as well as for operated and unresectable tumors. The technique achieved moderate local control (64% at 4 years) similar to other radiotherapy methods. Tumors of size more than 5 cm and those in the scalp are at higher risk for recurrence. Szlag et al. [17] and Wittych et al. [18] have also described the use of HDR-BT surface mould in the management of angiosarcomas of the scalp in elderly patients. Angiosarcomas are more aggressive tumors compared with DFSP, and patients in both the reports suffered local recurrences. Similarly, Ruiz et al. [19] have described the use of surface mould HDR-BT in the management of 3 cases of Kaposi’s sarcoma. The treatment modality was described as simple, safe, quick, and non-invasive, with a complete response in all the treated cases. These reports highlight advantages of the use of surface mould HDR-BT, such as short treatment time, easy tolerability, high compliance combined with conformal targeted dose distribution, and a low integral dose, which are evident in our case also. The only difference in our report was the use of this modality in the treatment of DFSP.
Only one previous report by Bandyopadhyay et al. has described the use of this modality in the treatment of DFSP of the scalp [20]. Compared with our study, the patient in this study was a younger, 24-year-old female and the tumor size was smaller, but the deep margin of tumor was positive for cancer cells. Except for a smaller PTV and a larger catheter separation (1.5 cm), the technique and dose used were similar to the current study, including local control and minimal late toxicity, which were the same in both the research. Therefore, two independent successful experiences provide good quality evidence for the use of this modality in this clinical scenario.
Conclusions
Surface mould HDR-BT, as a modality of delivering radiotherapy in superficial sarcomas in superficial regions, such as the scalp, remains an underutilized, yet safe, effective, logistically feasible, and well-tolerated treatment option. Due to rarity of the cases, it should be explored further in multi-institutional settings to identify different pathologies and clinical circumstances, in which this technique can be used successfully.



