Purpose
Endometrial cancer is one of the most prevalent gynecological cancer, with an incidence rate of more than 380,000 patients per year, and is responsible for approx. 90,000 deaths per year worldwide [1]. The median age of patients is around 60-65 years, and majority of patients are diagnosed at an early stage [2]. Early-stage patients, who underwent total abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO) have a five-year overall survival (OS) of around 95% [3]. In addition, patients may need to receive adjuvant therapies according to certain indications, including high-grade, deep stromal, myometrial, and lymphovascular invasion, lymph node(s) metastasis, and margin involvement [4-6].
According to PORTEC-1 (post-operative radiation therapy in endometrial cancer) trial [4, 7] and GOG 99 (Gynecological Oncology Group) trial 99 [5], external beam radiation (EBRT) demonstrated utility in high-intermediate-risk patients (stage IB grade 1, any patients with grade 2, and stage IA grade 3 were considered as high-intermediate-risk) in terms of reducing local failure; however, OS was not shown to improve. PORTEC-2 trial [8] with high-intermediate-risk patients without staging pelvic lymphadenectomy (LND), showed no difference between high-dose-rate brachytherapy (HDR-BRT) and EBRT in vaginal cuff recurrences. However, EBRT was demonstrated to be superior in pelvic control in comparison with HDR-BRT alone. Ten years follow-up of PORTEC-2 trial confirmed that vaginal cuff BRT could be the standard of treatment for high-intermediate-risk early-stage endometrial cancer [9]. Moreover, in advanced cases, additional use of EBRT in HDR-BRT is recommended because of reduced pelvic recurrences in EBRT and BRT [10].
As shown in PORTEC-2, vaginal cuff brachytherapy in endometrial cancer patients had excellent therapeutic outcomes [8]. Application of a single-channel vaginal cylinder to cover a 5 mm depth of vaginal mucosa is a simple and effective technique. Therefore, it became the most popular instrument to perform HDR-BRT [11]. Worldwide, the most common dosimetry method in patients who had undergone HDR-BRT is two-dimensional (2D) technique. According to physical examination, length and diameter of appropriate vaginal cylinder should be considered [12]. Since there is no cross-sectional anatomy imaging, dosimetry of target volume and OARs are impossible. In contrast, in image-based three-dimensional (3D) planning, a treatment is designed according to contours delineated and dose constraints. There are few studies comparing 2D vs. 3D planning in HDR-BRT of endometrial cancer. Kim et al. [13] compared 2D and 3D planning in endometrial cancer treated with HDR-BRT. They planned 2D plans through manual optimization points and also planned 3D in CT images. They revealed that all determinants, such as D90, D100, V100, V150, and V200 were lower in 3D comparing to 2D. OARs, including rectum and bladder received lower doses in 3D compared to 2D. Therefore, this study showed that 3D has superiority over 2D in terms of saving OARs without compromising target volume dose. A study by Humphrey et al. [14] demonstrated that CT scan can help in cylinder reposition to reduce air gap in roughly 7% of patients. Another study by Russo et al. [15] focusing on OARs (bladder and rectum) showed that dose calculation in 2D plans according to ICRU (International Commission on Radiation Units and Measurements) points could not accurately estimate delivered dose. It was shown that maximum dose and D2cc calculated in 3D plans were more accurate than ICRU dose, and consequently, the authors concluded that ICRU could not represent dose delivered to OARs. Recently, Gultekin et al. [16] reported that 3D planning in HDR-BRT of endometrial cancer patients could spare OARs more than frequently used methods, which deliver prescribed dose to a 5 mm distance from the surface of cylinder or at its’ surface.
The most popular source applied in gynecologic brachytherapy was iridium-192 (192Ir), due to its small size and suitable physical aspects, until miniaturized cobalt-60 (60Co) was introduced into clinical practice. Here, the advantages and disadvantages of 60Co administration will be compared to 192Ir [17]. First, a longer half-life of 60Co (5.3 years) in comparison to 192Ir (74 days) resulted in reduced operating costs, with logistical and economic advantages [18-21]. For example, in a definite interval time, 25 source replacements of 192Ir would be needed in comparison to just a single application of 60Co [17]. Second, despite the fact that these two sources could have different physical characteristics [17], studies showed that dose distribution is identical. Also, toxicity and clinical outcomes of 192Ir and 60Co were similar. However, there is a limitation regarding administration of 60Co, which emits high-energy gamma rays, and that requires the staff to provide a thicker shielding material [17].
There are few studies that compared 2D and 3D planning with different methods of dose calculation in 2D plans, including ICRU point [15], manual placing of optimization point [13], dose delivery to a 5 mm depth of vagina wall, or dose delivery to the surface of cylinder [16]. In the current study, a 5 mm depth of the vagina was chosen as dose delivering point in 2D planning, because this depth is comparable with vagina thickness contoured in 3D planning, and at the same time, the bias of comparing PTV coverage between 2D and 3D plans was limited. To the authors’ knowledge, the present study is the first research to compare 2D with 3D planning through administration of 60Co. The aim of this study was to determine whether 3D plans provided better target coverage, and to explore differences in dose delivered to OARs, when considering cross-sectional imaging and contours in 3D planning compared to 2D controls using 60Co as a brachytherapy source.
Material and methods
Population
This cross-sectional study was conducted in radiation oncology departments of two different hospitals. Healthcare professionals and treatment protocols of both hospitals were the same, as both of them belong to one university. According to Kim et al. [13] who considered dose delivered to 1 cc of rectum in 2D plan (88%) and 3D plan (93%), acceptable difference was considered as 15%, first error was less than 5%, and power (1 – beta) was 90%, with thirty-five patients analyzed through non-inferiority sample size formula [22]. Forty-one confirmed cases of early-stage endometrial cancer of IA to IIB FIGO stages were enrolled, and underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO) and subsequent radiotherapy using HDR-BRT as monotherapy. All the patients were provided with written informed consents, and their data were coded and kept confidential. Furthermore, study protocol was approved by an ethics committee of affiliated university.
CT scanning and contouring
The patients underwent a non-contrast pelvic CT scan (Sommatom DR®, Siemens, Germany), with slices thickness of 3 to 5 millimeters from mid-pubis to the lower border of ischial tuberosity. Patients with sub-total hysterectomy or those with cervical tissue remnants in CT images were excluded from the study after scanning. Vagina was considered as the target volume. Rectum, sigmoid, and bladder were delineated as OARs. Vaginal length for clinical target volume (CTV) was defined according to grade and histology of the tumor. CTV was contoured superiorly 2.5 cm upper cylinder, and could be modulated with vagina in CT images [13]. Patients with grade 1 and 2 endometrioid carcinoma were treated with a 4 cm length cylinder, if ≤ 50% of myometrial invasion and a 5 cm length cylinder in case of > 50% myometrial involvement. Six centimeters of the vagina were treated in grade 3 endometrioid carcinoma, clear cell carcinoma, and serous papillary carcinoma [12]. In addition, thickness of target volume was contoured based on CT scan slices (vaginal width was considered around 5 mm and lessened based on adjacent OARs, or increased according to thickness seen on CT slices), and length of the vagina was modulated based on clinico-pathological aspects.
The optimum plan was described when the target volume received more significant percentage of the prescribed dose, while dose delivered to OARs was as little as possible. The upper and lower borders of the rectum were set at S2-S3 interspace and puborectalis muscles, respectively. In addition, the sigmoid was contoured from true pelvis to recto-sigmoid junction, and the lower border of bladder was also considered as levator ani muscle. Contours were outlined by a physician and then confirmed by another physician and a physics master.
2D vs. 3D planning
After these pre-requisites, the treatment was planned using HDR Plus v. 8.2.3® software and administration of TG-43 algorithm, according to delineated contour and physical examination considering length of treatment, cylinder diameter, and prescribed dose. Both 2D and 3D techniques were used for treatment design. The physics master was blinded to 2D plan when performing 3D design in relation to contour and optimized dose based on the best coverage of CTV and OARs’ constraints. Moreover, 2D planning was performed to deliver the prescribed dose to a 0.5 cm depth of the vagina, and optimized when 150% isodose was located at the cylinder surface and 200% inside the cylinder. This type of planning was performed without any imaging after which, a defined dwell time on 2D planning was performed on CT images contoured as previously described. Dwell points with a 3.5 mm distance on 2D plans were maintained in all cases, while their data were transferred to CT images. Therefore, 2D plan was copied on CT images, and dose constraints were consequently measurable. The patients received 4 to 7 Gy in 3 to 5 fractions, with one-week interval, using the same treatment plan designated for the first fraction. HDR Plus v. 8.2.3® software, TG-43 algorithm for dose calculation, and 60Co source (MultiSource, Eckert-Ziegler®, Belgium) were used for brachytherapy.
Statistical analysis
D90 and D99 (defined as the dose delivered to 90% and 99% of the target volume, respectively) as well as V100, V150, and V200 (defined as the volume of target, which received 100%, 150%, and 200% of the prescribed dose) were considered. D0.1cc, D1cc, and D2cc (defined as the dose received by 0.1, 1, and 2 cc of an OAR) were also calculated to evaluate OARs dosimetry. Data were recorded in SPSS software version 16 and compared between 2D and 3D plans using Kolmogorov-Smirnov test to check normality. Moreover, a parametric test (paired sample t-test) and non-parametric test (Wilcoxon ranks test) were applied to compare variables between 3D and 2D plans. Mean, median, standard deviation, and interquartile range were calculated for all quantitative data, and frequency and percentage of qualitative data were assessed. P-values under 0.05 were considered significant.
Results
Population
In total, 41 patients with stage I-II endometrial cancer were enrolled; however, three cases were excluded due to findings of cervix residue on CT scans, and another patient was excluded due to a failure in attending CT scan. Finally, 37 patients were enrolled in the present study, with 15 (40.5%) stage I and 22 (59.5%) stage II cases. The mean patients’ age was 62 ±3.5 years. Twenty-six patients (70.3%) were grade 1/2, and 11 patients (29.7%) were grade 3. Both 2D and 3D plans were designed with equal dose according to a physician’s order, and the mean and median vaginal length, target volume, and prescribed dose in each fraction were 51.09 ±2.3 mm, 49.1 ±2.1 mm, 23.83 ±1.9 cc, 21.7 cc, and 5.95 ±0.11 Gy, and 6 Gy, respectively.
Comparing parameters in 2D vs. 3D plans
Figure 1 shows the target volume, OARs, and isodose lines in 2D and 3D plans in one patient. First, parameters of the target volume dosimetry were analyzed. Our results showed that the mean amount of both D90 and D99 were significantly higher in 2D plans comparing with 3D plans. Furthermore, although V100 showed no significant difference between 2D and 3D plans (p > 0.05), V150 and V200 were considerably higher in 2D plans compared to 3D plans. Table 1 shows a comparison of D90, D99, V100, V150, and V200 between 2D and 3D plans.
Fig. 1
Target volume, organs-at-risk (OARs), and isodose lines in 2D and 3D plans. The bladder, rectum, and sigmoid are contoured with yellow, pink, and blue lines, respectively. Activated sources and target volume are shown with red color. Isodose 100 is purple, which is tangential to the target volume in 3D; however, this line is beyond the target volume in 2D. In addition, as it is shown in the pictures, this line passed a lesser volume of OARs in 3D plans
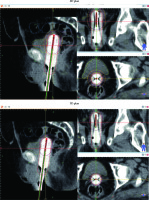
Table 1
Comparison of D90, D99, V100, V150, and V200 between 2D and 3D planes
Second, parameters suggesting the delivered dose to OARs, including D0.1cc, D1cc, and D2cc were compared through a non-parametric test between the two groups of patients. As shown in Table 2, all parameters regarding dose delivered to the rectum and sigmoid were lesser in 3D than 2D plans (p-value < 0.05). The dose delivered to the bladder analyzed by the parametric test was lesser in 3D plans; however, D0.1cc was not statistically significant (p-value > 0.05), which means that 3D planning could cause the rectum sigmoid and bladder to receive a reduced amount of dose.
Table 2
Comparing Dmax, D0.1cc, D1cc, and D2cc in rectum, sigmoid, and bladder in 2D and 3D planes
Discussion
High-dose-rate BRT plays a significant role in adjuvant treatment of early-stage endometrial cancer [8, 9]. Although radiation therapy reduces the risk of vaginal cuff relapse, it does not impact overall survival [8]. PORTEC-2 trial was conducted to find practical and less toxic treatment method, and reported that HDR-BRT could be used as the standard of treatment, since it can reduce the risk of local relapse as well as EBRT [8]. HDR-BRT could be performed through a BRT cylinder, single-channel, or multi-channel cylinder. A single-channel cylinder is the most common way to cover the vagina. However, a multi-channel cylinder can spare normal tissue more than a single channel due to its’ feasibility of deactivating sources near OARs [11, 23, 24]. As early-stage endometrial cancer patients treated with single-channel HDR-BRT would experience a prolonged survival, administration of a less toxic treatment method is essential. Using CT scan imaging in 3D planning to reduce toxicity of HDR-BRT was evaluated in several studies. However in the present study, it was the first time that the thickness of target volume was contoured based on CT scan slices, and the length of vagina was modulated based on clinico-pathological aspects.
Globally, two-dimensional planning is the most common method of treatment. The reason for not using cross-sectional images in 2D plans was that dose gradient falls sharply around brachytherapy cylinder’s sources. Here, we challenged this idea to determine whether using CT scan images can improve dose delivered to target volume and reduce dose to OARs. This hypothesis should be examined by comparing dose received through 3D planning, which optimizes dose on contouring and plan designation, according to activity sources in 2D plan on CT scan images. As discussed in previous studies, there are different optimization methods in 2D plan, including normalization point in the lateral aspect of vagina, ICRU point, setting normalization point at the surface of cylinder, or at a 5 mm depth of vagina. In the present study, we applied 60Co in the treatment of our patients; optimized 2D plans were based on 5 mm depth of vagina and dosimetry parameters of 2D plan and 3D plan of each patient were compared.
A comparison of D90 and D99 between 2D and 3D plans has shown that although mean D90 and D99 were more in 2D than 3D plans, only six patients received D90 less than 90% in 3D plans compared to nine patients in the 2D group. Similar findings were reported by Kim et al. [13] who reported mean percentage of D90 at around 106% in 2D comparing with 103% in 3D plans. The same results were shown by a study by Gultekin et al. [16] who stated that D90, D95, and D100 were less in 3D plans compared to 2D plans, when normalization point was chosen in a 5 mm depth of the vagina. Moreover, Zhang et al. [25] showed that tumor cell distribution and shape of the target should be considered in radiation therapy designation due to radio-biologic effects. Based on our work and analyzing V100, V150, and V200, it was shown that V100 was approximately equal between 2D and 3D plans, even though V150 and V200 were less in 3D plans. This means that 3D plans not only could deliver the prescribed dose to more volume of the target volume, but also was able to spare more volume of the vagina from receiving an excess dose. Kim et al. [13] demonstrated that both V100 and V150 were less in 3D plans in comparison with 2D plans.
Another advantage of using CT scan imaging and 3D planning is that, since there were no organs’ sectioning in 2D plans, therefore, calculation and judgment of the delivered dose to target and OARs were impossible. Russo et al. [15] used ICRU point dose for bladder and rectum and could not accurately estimate dose delivered to OARs. They concluded that dose calculated through ICRU point was less than D2cc and Dmax in bladder and rectum. Kim et al. [13] and Gultekin et al. [16] also revealed that dose delivered to these organs, including D0.1cc, D1cc, and D2cc, were smaller in 3D plan rather than 2D plan.
Computed tomography (CT) scan imaging can yield information about the position and status of vaginal vault. In the present study, 3 out of 41 patients were diagnosed with residual cervix during CT scan imaging. They were excluded due to impossibility of covering the remained cervix through brachytherapy cylinder as well as the position of cylinder and potential air gaps between the cylinder and vaginal cuff. In a study by Humphrey et al. [14], CT scan imaging after cylinder placement could exhibit air gaps, which might be modified in a next imaging setup. They showed that CT scans could be used as a guide to choose the most fitting cylinder to occupy vaginal space. Similar findings were reported by Sikorska et al., where it was shown that CT scan could help choosing the best cylinder size, resulting in air gaps decreasing [26]. Another study with CT scan performed by Marcos et al. [27] at the Johns Hopkins University revealed that approximately half of patients need cylinder repositioning between HDR-BRT fractions, suggesting that CT scan imaging can guide physicians to place the applicator in the best position.
Although CT scan imaging-based planning has several advantages over 2D planning, it has some limitations. First, CT scanning is a time-consuming and expensive procedure, and may require additional workload in brachytherapy departments. Second, its power to discriminate soft tissues is lesser than MRI. Finally, the possibility of cylinder repositioning between fractions makes it necessary to repeat CT scanning in each fraction.
Since early-stage endometrial cancer is highly curable, particular attention should be paid to reduce treatment toxicities. This study demonstrated that 3D planning can deliver a suitable dose to the target while sparing OARs. Furthermore, it was the first time that 60Co was applied in a comparison of 2D and 3D planning, showing that 3D planning could be beneficial in developing countries.
Conclusions
The present study indicate that using CT scan imaging to perform brachytherapy planning can deliver suitable dose to the target volume while sparing OARs, especially rectum and sigmoid. In addition, imaging before dose delivery to target volume can reveal the position of cylinder in the vagina, and provide an information whether the cylinder is capable of covering the target.



