Purpose
Non-melanoma skin cancers (NMSCs) comprise largely squamous cell carcinoma (SCC) and basal cell carcinoma (BCC). The global incidence of NMSCs was estimated to be around 1 million in 2018 [1]. The NMSCs comprise 1-2% of cutaneous neoplasms in Indians, of which 83.9% were confirmed as SCC and 16.1% as BCC [2,3].
The primary treatment of choice for NMSCs is surgery. Radiation therapy (RT) can be used as a primary treatment for patients disqualified from surgery, patients in whom good cosmetic and functional results cannot be achieved by surgery or patients with lesions associated with clinical perineural invasion (PNI) with gross tumour extending to sites that render complete resection unlikely or unfeasible, such as the cavernous sinus [4]. Postoperative RT can be added in presence of adverse pathological features such as close or positive margins, PNI, cartilage or bone invasion, as it reduces local recurrences [5].
Radiation therapy in these cases can be delivered by a number of modalities consisting of high dose rate (HDR) brachytherapy (BT) or external beam RT (EBRT). Among EBRT techniques orthovoltage RT with beam energies in the range 100-250 kV or electrons or high energy photons can be used [5]. Among the above modalities, the most commonly used modalities are electrons and surface mould brachytherapy.
Electron beam therapy often requires templates and lead cutouts, the construction of which can be messy and uncomfortable for the patient when using skin collimation for better dose coverage. In the treatment of small fields, electron dosimetry requires special attention because the percentage depth dose (PDD) and output change significantly according to the specific block shape and size [6]. HDR BT, despite the more heterogeneous dose distribution, presents several advantages, particularly for irregular surfaces and challenging setups [6]. Reports of older published series mainly done in European facilities with wide experience in BT showing efficacy in treating skin cancer have led to recent interest and growth in new techniques [7,8,9]. Several innovative applicators have been introduced to the BT community, and the use of skin BT has significantly increased [10].
Surface applicators have been used in radiotherapy since the turn of the 20th century. Applicators such as wax and paraffin skin custom moulds were developed and used with radium needles or radon seeds to cure skin cancer [11]. The advantages of brachytherapy over electrons are a rapid dose fall-off outside the target volume and short duration of treatment [6,12,13,14,15]. Moreover, with respect to difficult areas such as in and around the nose or earlobe, major disadvantages of electron or EBRT are the setup errors and difficulty in planning in small, irregular fields and so a significant PTV margin must be given [12].
Although BT should be better logically, dosimetric studies comparing BT and electrons for treatment of superficial skin tumours, especially in difficult areas such as the nose, are lacking. Moreover, dosimetric studies of BT with 3D planning are even more scarce. The purpose of this study was to make a dosimetric comparison of computed-tomography based (CT-based) mould brachytherapy treatment plans with 3D conformal electron beam treatment plans in the treatment of NMSC of the head and neck region, especially in difficult sites over and around the nose.
Material and methods
Patient selection and patient characteristics
Patients with NMSCs (BCC or SCC) of the head and neck region, who received adjuvant radiation in the Department of Radiotherapy, Medical College and Hospital, Kolkata, between December 2017 and November 2018 with HDR BT using individual surface mould applicators, were enrolled for this study. The patients who were selected had received only HDR BT treatment to the local site as part of their adjuvant treatment and did not require any nodal irradiation.
A total of 10 patients were selected and analysed. The patients were aged between 40 and 75 years (median age of 56.6 years). Of the 10 patients, 6 patients had histologically proven basal cell carcinoma and 4 patients had squamous cell carcinoma. Out of the 10 patients, in 7 patients the lesion was located over the ala of the nose and adjacent cheek, in 2 patients the lesion was over the nose proper and in 1 patient the lesion was over the root of the nose. All of the patients had undergone radical resection followed by adjuvant radiation with HDR brachytherapy using individual surface mould applicators. Among the patients, 9 received adjuvant therapy owing to a close margin and 1 patient needed radiation due to evidence of cartilage invasion. None of the patients had any positive nodes or any evidence of distant metastasis at the time of treatment. None of the patients had indications for regional nodal irradiation.
Details of brachytherapy treatment received by the patients
The tumour bed was marked over the scar in correlation with preoperative images. An area beyond the bed with a 2 cm margin all around was marked as the target area (Figure 1). The patient was then immobilised using a three clamp head and neck thermoplastic mask. The target area was redrawn over the mask. On the thermoplastic mask layers of dental wax (of about 5 mm thickness) were applied to adequately cover the target area. Grooves were created over the wax with 1.5 cm separation and flexible HDR catheters of adequate length were then fixed to the mould, one on each of these grooves. Additional layers of dental wax were applied to cover the catheters and to give stability to the mould (Figure 2). To ensure proper dose delivery on the borders of the target, the number of catheters was selected to cover the whole target volume with the margin.
Fig. 1
Schematic diagram showing the relation of tumour bed, target area and the arrangement of plastic catheters
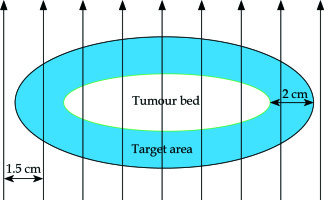
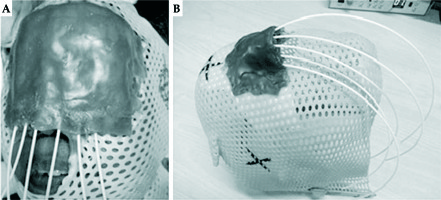
Fig. 2
Thermoplastic masks with customised moulds with plastic catheters in situ after preparation and before simulation on a patient (A) and without the patient (B)
Before acquiring the CT scan, the target area was marked with thin copper wire. CT scan (BrillianceCT-16 slice with Accusim Virtual simulation; Philips Health Care Solutions, DA Best, The Netherlands) was performed using a thin slice width of 1-2 mm from the vertex to just below the chin (120 kV and 250 mAs). After CT simulation the data were transferred to the treatment planning system (TG-43 compliant BrachyVision TPS, Eclipse Version 13.5, Varian Medical Systems, Palo Alto, CA, USA) computer. Then following image reconstruction, the planning target volume (PTV) corresponding to the target area was contoured. The skin and soft tissue underlying the target area formed the depth of the PTV. A small additional margin was taken for the PTV to account for setup errors. The organs at risk (OARs: underlying bones, eyeballs, lens and skin) were also contoured and the dose was prescribed. After that, optimisation was done such that the 100% isodose line conformed to the PTV as much as possible.
The total dose prescribed was 42 Gy to the 100% isodose with an equivalent dose in 2 Gy (EQD2) of 47.25 Gyα/β10, delivered in twice daily fractions of 3.5 Gy each at least 6 hours apart for 12 fractions. The scheduled duration of treatment was 6 days. Treatment was delivered using iridium-192 (192Ir) with the GammaMed plus HDR afterloader unit (Varian Medical Systems).
The brachytherapy treatment plans of the selected patients were then analysed and various parameters (details given later) were recorded to evaluate the dose coverage, conformity and normal tissue sparing.
Treatment planning for electron beam therapy
The CT scans acquired for brachytherapy planning were transferred to the treatment planning system (Eclipse Version 15.5, Varian Medical Systems, Palo Alto, CA, USA) for electron beam planning. The primary brachytherapy bolus density was removed (changed to air equal density). For electron planning, the bolus was virtually added (Figure 3).
Fig. 3
Comparison of dose colour wash of customised surface mould brachytherapy plan and electron beam plan for a patient with NMSC over nasal bridge (A, B) and over the nasal skin (C, D)
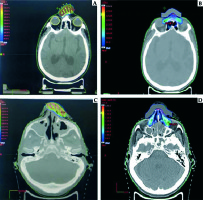
The PTVs for the brachytherapy plans were contoured similar to the PTV for the electron plans and no additional margins were given. The OARs were also contoured similar to those in the brachytherapy plans.
For each patient 2 or 3 electron beam plans (created using the Monte Carlo algorithm) using different energies (6, 9 or 12 MeV) and different bolus thicknesses (5 or 10 mm water density bolus) were generated and the best plans, with respect to optimal coverage of the targets and acceptable doses to the OARs, were selected for analysis.
Parameters similar to those recorded for the brachytherapy plans were also recorded for the electron plans.
Plan comparison
The PTV coverage was assessed by recording the minimal doses to 90%, 95% and 5% of the PTV (D90, D95, D5), volumes of PTV receiving 90%, 100%, 125%, and 150% of prescribed dose (VPTV90, VPTV100, VPTV125, VPTV150) defined as the percentage of the whole PTV volume, dose homogeneity index (DHI) and dose non-uniformity ratio (DNR) (only for BT plans). We calculated the conformal index for 90 and 100% of the prescribed dose (COIN90, COIN100). For OARs, minimum doses to the most exposed 2 cc and 0.1 cc of the organs were noted. The OARs for which the doses were recorded were underlying bones, eyeballs, lens and overlying skin.
All doses were expressed as a percentage of the total dose. The prefix BT or Electron before the name of the parameter applies to the BT or Electron plan. The following definitions for parameters were used:
where V90, V100 and V150 are the volumes of PTV receiving 90%, 100% and 150% of the prescribed dose respectively.
Data analysis
The normal distribution of the data was analysed using the Shapiro-Wilk test. For comparison of the data, the t-test for paired samples or Wilcoxon’s test was used depending on the normality of the data. All statistical analyses were performed using IBM SPSS v23. Results were considered significant if the p value < 0.05.
Results
The results are summarised in Tables 1 and 2.
Table 1
Dosimetric comparison of the customized surface mould brachytherapy and electron beam plans
Table 2
Comparison of dose volume parameters of the organ at risk, between the surface mould and electron beam therapy plan
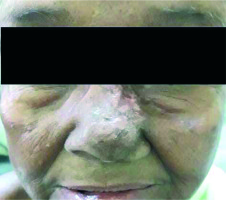
For BT plans, the average D0.1cc for overlying skin was 171.8% (SD 19.7%). We tried not to exceed 200% of the prescribed dose to the most exposed 0.1 cc of skin surface inside the PTV. This goal was achieved in all patients. Because of this very high dose to the skin, most of the patients developed significant acute and late skin toxicities (Figure 4). For Electron plans, in no patient did the dose to the most exposed 0.1 cc of the overlying skin inside the PTV exceed 200% of the prescribed dose.
Fig. 4
Post-treatment skin hypopigmentation. 12 weeks after being treated with surface mould brachytherapy with hypopigmented area corresponding to skin surface receiving > 150% of the prescribed dose
Comparison of brachytherapy and electron plans
We observed no significant differences in D90 and D95 between the BT and Electron plans, but there was a significantly higher mean D5 for the BT plans (p < 0.05). No significant differences were found in the mean VPTV90 and VPTV100 but the mean VPTV125 and VPTV150 were significantly higher in the BT plans (p < 0.05 for both). We found no difference in COIN100 but a significantly higher value was observed for COIN90 for the BT plans (p < 0.05).
For OARs, significantly higher doses were observed with Electron plans for most of the OARs except for D0.1cc bones and D0.1cc lens (no significant differences in these two). As for skin doses, the D0.1cc was significantly higher for the BT plans (p < 0.05).
Discussion
In the literature, not many studies are present which deal with treatment of NMSCs of the nose and surrounding difficult areas with brachytherapy. In some of them, special applicators (e.g. Valencia, Leipzig, etc.) or electronic brachytherapy were used [19,20,21]. But they were not feasible for irregular surfaces. Experiences of using custom mould brachytherapy are also scarce. A few studies have used them for treatment of skin cancers and the results have been promising, compared to other modes of radiation delivery [13,15,22,23,24]. In a few studies, 3D planning based custom mould brachytherapy was used and they showed very good dose distribution with respect to PTV coverage and OAR sparing. In one of the studies [25], surface mould based HDR brachytherapy showed very good results for cutaneous facial BCC and SCC but also showed high failure rates in tumours located over the nose. This emphasizes the inherent difficulty in treating lesions over small irregular surfaces.
Now coming to electrons as a modality of treatment of superficial skin cancers, especially on small irregular surfaces, the difficulty is that there is huge variation in the PDD and output parameters and they depend on the field size and the electron energy used [26]. Studies comparing electrons with surface mould brachytherapy for treatment of NMSC are rare. The studies [27,28] which compared these two modalities directly concluded that local control, acute and late toxicities were comparable but brachytherapy had the advantage of better conformity, shorter treatment times and cost effectiveness. However, in another study [29], brachytherapy had better cosmesis compared to EBRT. One study [17], which compared surface mould brachytherapy with EBRT, concluded that computed tomography-based surface mould brachytherapy for superficial lesions on irregular surfaces is superior to 3D-CRT EBRT in those locations, in terms of conformity and normal tissue sparing ability in medium to high doses.
In our study, we found very good PTV coverage with both electron and brachytherapy plan (as demonstrated by the non-significant differences in D90, D95, VPTV90 and VPTV100 between the two modalities). The overdoses inside the PTV for BT plans were acceptable (VPTV125 42%, VPTV150 15.8%) but they were still significantly higher than the electron plans. This translates into higher heterogeneity inside the PTV with BT.
This study showed that conformity was much better with brachytherapy (significantly different COIN90 values). This finding corroborates other studies [17,27]. However, the COIN100 values were not significantly different.
The homogeneity indices were calculated using different formulas for the two modalities. Therefore direct comparison of this parameter is not ideal and was not done. However, considering this parameter and taking into account the greater overdosages inside the PTV for the BT plans, we can say that there was significant heterogeneity in the BT plans.
OAR sparing was much better with the brachytherapy plans, although we did not use any lead shielding for the eyes in the Electron plans. This might have been a potential cause of much higher OAR doses in the electron plans. One very significant finding was the very high doses to the overlying skin inside the PTV with the brachytherapy plans (significantly higher D0.1cc doses). This can lead to severe acute reactions and even late skin toxicity. But considering the fact that these are superficial skin tumours, whether this finding is good or bad remains debatable.
Dose calculations were done using TG-43 formalism for the brachytherapy plans and the Monte Carlo algorithm for the electron plans. Both of them have some limitation with respect to small field dosimetry [26,30]. So errors in dose calculation could have affected the results.
This study was a dosimetric study only and the results need to be validated in prospective trials directly comparing these two modalities. Moreover, the sample size for this study is very small and this makes it difficult to draw definitive conclusions based on the results.
Conclusions
Surface-mould HDR BT might offer a better conformity index and coverage than electron EBRT for skin non-melanoma cancers of the nose, with better sparing of the lens, eyeballs, bone and cartilage at the price of higher maximum skin dose, which can be relevant for skin toxicity, even for adjuvant doses. Prospective clinical studies are needed to validate our small sampled dosimetry results. However, data comparing these two modalities are limited and this study aims to provide a small insight into this topic.



