Purpose
Transurethral resection of the prostate (TURP) is a surgical procedure performed under general or spinal anesthesia to remove the prostate tissue proximal to the verumontanum and distal to the bladder neck as treatment for urinary obstruction. The surgeon removes as much tissue as necessary without penetrating the prostatic capsule to allow the patient to void normally [1].
Low-dose-rate (LDR) 125I seed brachytherapy is an established treatment for men with localized low- and selected intermediate-risk prostate cancer. Both the American Brachytherapy Society (ABS) recommendations on permanent seed implant [2,3] and the Groupe de Curiethérapie-European Society for Radiotherapy and Oncology (GEC-ESTRO) guidelines [4] consider prior TURP as a relative contra-indication for prostate permanent seed brachytherapy. In many experienced brachytherapy centers, a history of endoscopic resection of the prostate remains an absolute contra-indication to prostate brachytherapy, which is reflected in small number of publications in this area [5]. However, with more extensive experience, optimization of imaging techniques, and improved loading and dosimetry techniques, the complication rate in this group of patients appears low. More recent reports [6,7,8,9] dealing with this issue suggest that brachytherapy can be safely performed in a post-TURP patients’ group assuming modern imaging and optimized dosimetry techniques used. Unfortunately, the experience remains limited; reports are restricted and deal with small groups of patients. Against this background of early reports in the literature and further personal experience, the GEC-ESTRO Uro-GEC group developed a protocol for consistent target and organ at risk contouring, definition of clinical target volume (CTV), and dosimetric parameters, based on the GEC-ESTRO guidelines for prostate seed implantation [10], but with some specific adaptation for the post-TURP situation. Pilot data from the use of this protocol in a small cohort of patients has been presented [9] as well as a larger multi-center prospective planning study [11]. Due to wide range of different implantation techniques available in this multi-center environment, the variability between institutes has also been evaluated [11]. This feasibility planning study confirmed that in a multi-center setting and experienced brachytherapy units can achieve the dose constraints defined in this modified protocol for patients undergoing prostate post-TURP brachytherapy. With this reassurance, the Uro-GEC group of GEC-ESTRO proceeded with a prospective multi-center clinical study, in which patients were treated using this protocol with close evaluation of post-implant urinary function.
Material and methods
Selection of patients
Men with histologically proven organ-confined, low- to intermediate-risk (D’Amico risk group definition) localized prostate cancer, defined as clinical stage T1c-T2b, N0, ISUP grade group 1, 2, or 3 (Gleason score 6 or 7), with prostate specific antigen (PSA) level < 20 ng/ml, and with a history of transurethral resection (TURP) performed at least 3 months before brachytherapy procedure were enrolled in this multi-center prospective clinical trial. Other inclusion criteria were a prostate volume measured on transrectal ultrasound of less than 50 ml, the presence of a rim of prostate tissue of at least 1 cm around the post-TURP urethral defect at the postero-lateral sides of the prostate, the absence of significant TURP-induced urinary incontinence, and an international prostate symptom score (IPSS) less than 15. The use of neo-adjuvant anti-androgen hormonal treatment was permitted to downsize the prostate volume or to cover waiting time until the brachytherapy procedure. No adjuvant hormonal treatment was permitted. General inclusion criteria were a WHO performance status 0-1, the absence of contra-indication for anesthesia, no prior history of irradiation of the pelvis, the absence of other oncologic malignancy except adequately treated basal cell carcinoma of the skin or other malignancy, from which the patient was disease-free for at least 5 years. All patients provided informed consent to participate. The log-rank test was used to compare biochemical non-evidence of disease (bNED) between the subsets of patients analyzed.
Treatment and follow-up
All patients received transperineal ultrasound-guided LDR 125I seed brachytherapy monotherapy according to the institute’s usual practice and technique. Intra-operative interactive planning was recommended to ensure optimal accuracy of seed placement during the procedure. No supplemental external beam was allowed. Target and organ at risk contouring, definition of CTV, and dosimetric parameters had to follow the modified GEC-ESTRO guidelines for permanent seed implants as described previously [11]. Follow-up was scheduled every 3 months for the first year, then every 6 months, and included a physical examination, PSA, and prospective physician assessment of toxicity.
Study endpoints and statistical evaluation
The primary endpoint was the incidence of post-implant urinary incontinence, which was defined as the need to use at least one pad per day. Secondary endpoints were the incidence of acute and late urinary toxicity, the incidence of gastro-intestinal side effects, and the impact on sexual function, all evaluated using the common terminology criteria for adverse effects (CTCAE) version 4.0, and the freedom from biochemical failure, defined as time from implant to date of PSA failure (Phoenix definition, nadir + 2 ng/ml). The probabilities of freedom from biochemical failure and bPFS were estimated using the Kaplan-Meier method. To account for benign PSA bounce, patients were not considered to have PSA failures in case of a rise of the PSA by < 2 ng/ml during the first 24 months, followed by a subsequent non-hormonally induced PSA decrease. Late toxicity was defined as any toxicity occurring at > 12 months after implant. The 12-month cut-off was chosen on the basis of the half-life decay of 125I, 12 months being roughly 6 half-lives.
Results
In all, 99 patients were entered in this prospective multi-center study. All patients received a permanent seed implant in one of the six participating centers between March 2009 and June 2015. The median follow-up was 49 (range, 24-96) months. Patient characteristics are presented in Table 1.
Table 1
Patient characteristics
Acute and chronic urinary toxicity
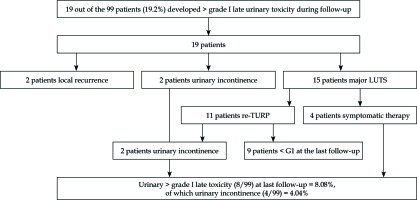
Moderate increase in urinary irritation occurred in the first three months after the treatment. None of the patients developed a greater than grade I acute urinary toxicity. In addition, no acute urinary retention was seen. 19 out of the 99 patients (19%) developed a more than grade I late urinary toxicity during follow-up. Further evaluation of these 19 patients showed that in 2 patients, the complaints were related to a local recurrence, 2 patients presented with late onset urinary incontinence, and 15 patients had major lower urinary tract symptoms. Four out of these 15 patients were successfully treated with symptomatic medication only; however, 11 patients underwent a re-TURP leading to a definitive re-TURP rate in this population of 10% (11 out of 99 patients). In 4 patients, this re-TURP was performed at 1-year, in 2 patients at 18-months, in 3 patients at 2-year, and in 2 patients at 4-year post-BT. Two out of these 11 patients who underwent a re-TURP developed urinary incontinence, whereas 9 patients reported a clear relief of the lower urinary tract symptoms. At last follow-up, only 8 out of the 99 patients (8%) still reported greater than grade I late urinary toxicity. Two of the 99 patients (2%) developed urinary incontinence after the brachytherapy procedure, and 2 patients (2%) developed urinary incontinence after brachytherapy and re-TURP procedures. The total urinary incontinence rate at last follow-up is thus 4% (Figure 1). The percentage of re-TURP varied between different participating centers. Evaluating the 4 major contributing centers, the incidence of re-TURP in center 1 was 10% (1 out of 10 patients), in center 2 it was 3% (1 out of 29 patients), in center 3 it was 11% (3 out of 27 patients), and in center 4 it was 28% (7 out of 25 patients).
Lower gastrointestinal toxicity
The observed lower gastrointestinal toxicity was extremely low. Some patients developed temporary grade I side effects. Only 1 out of the 99 patients (1%) developed a grade I late gastrointestinal toxicity during follow-up. Additionally, no patients presenting with perineal pain were reported.
Oncological results
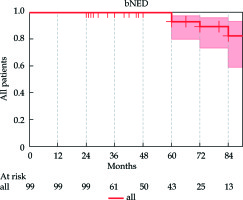
For the entire cohort of 99 patients, 7 patients (7%) presented a biochemical recurrence. Two of these patients presented with a local recurrence, whereas 5 patients developed distance metastases. The overall and cancer-specific survival was 100% at 5 years. The 4- and 5-year bNED were respectively 100% and 93% for the whole cohort (Figure 2).
The actuarial bNED with ISUP grade groups was 93.8% and 90.9% for patients with ISUP grade group 1 and 2 (Gleason score of 6 and 7), respectively (p = 0.393). Tumor stage was not significantly associated (p = 0.974) with biochemical control, neither was initial PSA or risk group (p = 0.56).
Discussion
The incidence of urinary incontinence in patients treated with brachytherapy following TURP is low at 4%. Overall, there was a low incidence of acute and late urinary and gastro-intestinal toxicity combined with excellent oncological results. These results are in concordance with the experience of other institutions [12,13,14, 15,16,17] and results seen in a non-TURP population [18,19,20,21,22,23].
In our series, the incontinence rate was 2% after TURP + BT and 2% in case of TURP + BT + re-TURP, with a total urinary incontinence rate of 4%.
Both the ABS recommendations on permanent seed implant [2,3] and the GEC-ESTRO guidelines [4] consider prior transurethral resection of the prostate (TURP), a relative contra-indication for prostate permanent seed brachytherapy. These recommendations were principally based on an early report from the Seattle group [5], describing their initial experience, and reporting a major risk of significant toxicity and urinary incontinence in brachytherapy patients who had undergone prior TURP. This study indicated a 17% risk of urinary incontinence.
However, subsequently, Wallner et al. reported only a 6% incontinence rate at 3 years in 19 patients treated with prostate seed implant after previous TURP, and there have been a number of retrospective reports of post-TURP brachytherapy with low urinary toxicity rates [6,8,9,24,25,26]. These are shown in Table 2.
Table 2
Literature list
| Publication | Number of patients | Urinary incontinence rate |
|---|---|---|
| Wallner et al. (1997) [6] | 19 | 6% (at 3 years) |
| Moran et al. (2004) [8] | 171 | N.A. |
| Claros et al. (2009) [24] | 16 | 0% (average follow-up 30 months) |
| Salembier et al. (2009) [9] | 51 | 0% (mean follow-up 18 months) |
| Latorre et al. (2013) [25] | 56 | 1% (mean follow-up 100 months) |
| Prada et al. (2016) [26] | 57 | 1.7% (mean follow-up 104 months) |
A further feature of this series is the number of patients undergoing a repeat TURP procedure after brachytherapy. The precise indications for this have not been collected. They may represent patients with urethral stricture or benign regrowth, but it is reassuring to note that the likelihood of incontinence in this group is no higher than the patients who did not need further intervention.
The strength of these results is that they are likely to reflect real world practice across different centers in different countries. The detailed dosimetric guidelines are an important component of this and should be closely followed in post-TURP patients. These patients have alternative options for treatment including prostatectomy or external beam radiotherapy; this data suggests that the risk of incontinence with LDR brachytherapy after TURP is no greater than either of these alternatives. Unfortunately, data on sexual function was incomplete and is not sufficient to provide a useful estimate. Similarly, we cannot comment on the need for ongoing medication such as alpha blocking drugs to maintain urinary function after brachytherapy.
Our study is the first multi-center prospective study evaluating the acute and late urinary and gastro-intestinal tolerance, the incontinence rate as well as the oncological outcomes in patients treated by TURP, followed by a prostate seed implant. It has been shown that brachytherapy for patients with a prior TURP is not only effective, but also with low induced toxicity with careful patient selection, at least 3 months from TURP and seed implant, using dosimetry adapted for the post-TURP anatomy.
Conclusions
With the present prospective data, added to the existing retrospective literature, and with more experience in the field as well as the optimization of the imaging techniques and improved loading and dosimetry techniques following the adapted guidelines concerning the specific post-TURP situation, prostate seed implants can be performed with excellent oncological results and low urinary and gastro-intestinal toxicity.