Purpose
High-dose-rate (HDR) brachytherapy offers several advantages over external radiotherapy techniques, such as a rapid dose fall-off at distances from the source, thus limiting dose exposure to surrounding normal tissues [1]. There are two main forms of brachytherapy: intra-cavitary brachytherapy that involves delivering a radioactive source to a space near the tumor; and interstitial brachytherapy, where a radioactive source is inserted into the body tissue. In HDR brachytherapy, the radioactive source is transferred to multiple pre-defined dwell positions with a remote afterloader [2-4].
Dose calculations for most commercially available brachytherapy treatment planning systems (TPSs) are based on the TG-43U formalism of the American Association of Physicists in Medicine (AAPM) [5] as well as the recommendations of the High Energy Brachytherapy Source Dosimetry (HEBD) Working Group [6]. In the TG-43U dose calculation formalism, the contribution of transit dose, i.e., the dose delivered during the transition of the source between dwell positions, is disregarded [7], whereas the transit dose component could be relevant. The transit dose mainly depends on transit speed and transit time of the source between dwell positions. Therefore, the existing studies have attempted to clarify the transit time, transit speed, and transit dose using thermoluminescent dosimeters [7], films [8], ionization chambers [9], video cameras [10-12], diodes [13], and Monte Carlo simulations [14].
Nevertheless, the necessity of transit dose correction remains unclear, as the impact of transit dose, particularly in clinical practice, has not been comprehensively investigated. The dosimetric impact of transit dose is affected by various factors, including the number of channels and active length, source strength, prescribed dose, and brachytherapy technique (i.e., intra-cavitary or interstitial). Therefore, a comprehensive evaluation of the dosimetric impact would offer a rough indication for clinical practice. Fonseca et al. [15] attempted to evaluate the dosimetric impact of transit dose in HDR prostate brachytherapy using Monte Carlo simulation. However, this technique is time-consuming and unsuitable for a comprehensive analysis.
In our previous study, we assessed the iridium-192 (192Ir) source status and determined the transit speed, transit time, and transit dose using a high-speed camera [11]. Linearity between the reciprocal number of dwell times and the increment of relative dose presented a potential formalization for the calculation of transit dose by introducing the effective transit time at inter-dwell positions. As the TG-43U dose calculation formalism can be used to compute dose distribution based on source positions, source strength, and dwell times, the effective transit time and inter-dwell position can be implemented in transit dose calculation using a commercially available brachytherapy TPS within a reasonable time. To comprehensively evaluate the dosimetric impact of transit dose on prostate and gynecological cancers, the transit dose component was investigated by using the effective transit time with a commercially available TPS in various brachytherapy techniques, such as intra-cavitary, interstitial, and hybrid brachytherapy.
Material and methods
Patients’ characteristics
This study was approved by the institutional ethics review board in National Cancer Center Hospital (approval number: 2017-091), and included 48 patients, who underwent HDR brachytherapy between April 2019 and January 2021. Table 1 summarizes the treatment information of the 48 cases: 12 prostate cancer patients treated with interstitial technique (PInt), and 36 gynecological cancer patients, including 9 cases treated with a cylinder applicator (GC), 9 treated with a tandem and two-ovoid applicators (GTO), 8 treated with interstitial needles (GInt), and 10 treated using a hybrid technique with tandem and ovoid applicators and interstitial plastic needles (GH). Medians (range, minimum-maximum) of the number of interstitial needles for GH, GInt, and PInt were 2 (1-9), 9 (5-12), and 18 (10-19), respectively.
Effective transit time
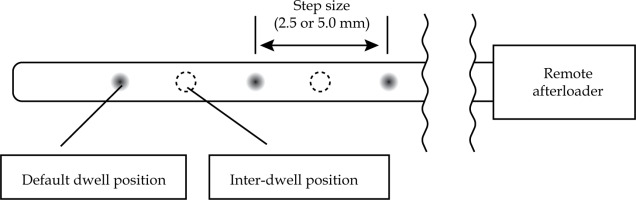
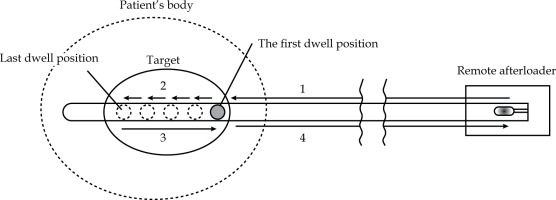
As shown in Figure 1, source movements were classified into four categories: 1. From remote afterloader to pre-defined proximal first dwell position in the target; 2. From pre-defined proximal first dwell position to distal last dwell position in the target; 3. From distal last dwell position to proximal end in the target; 4. From proximal end in the target to remote afterloader. Transit doses from movements No. 1 and 4 were not considered, as our TPS could only consider the transit dose inside patient’s body. Nevertheless, the dosimetric impact of transit dose outside of the patient’s body is limited because of inverse-square law. Therefore, we considered No. 2 and 3 situations in this study.
Fig. 1
Four categories of movements of the source: 1. From remote afterloader to pre-defined proximal first dwell position in the target. 2. From pre-defined proximal first dwell position to distal last dwell position in the target. 3. From distal last dwell position to proximal end in the target. 4. From proximal end in the target to remote afterloader
Regarding the transit dose, an effective transit time clarified in our previous study [11] at inter-dwell positions in commercially available TPS (Oncentra Brachy, version 4.5.3, Nucletron) was applied. Figure 2 shows a scheme for generating a transit dose-only plan by setting inter-dwell positions, which were between adjacent default dwell positions. For movement No. 2, inter-dwell positions at treatment were set between default dwell positions in original treatment plan in both cases of 2.5 and 5.0 mm step sizes. Therefore, the step size of inter-dwell position also reflected the step size of original treatment plan for each patient. For the transit dose from movement No. 3, settable dwell positions (2.5 mm step size) were activated for calculation, because the source was continuously returned to remote afterloader after distal last dwelling in the target.
The effective transit time of the source was estimated using the transit time measured with a high-speed camera and microSelectron mHDR-v2r (Nucletron, Elekta Company, Stockholm, Sweden), as depicted our previous study [11], after applicator reconstruction and delineation based on CT images. The effective transit times for 2.5 and 5.0 mm step sizes at inter-dwell positions were 13.7 and 20.5 ms, respectively. After distal last dwelling in the target, the source was retrieved by remote afterloader at relatively constant velocities of ~345 mm/s. At a step size of 2.5 mm, this speed corresponded to an effective transit time of 7 (2.5/345) ms (Table 2).
Table 2
Effective transit time as described in previous study [11]
| Step size (mm) | Effective transit time (ms) | |
|---|---|---|
| During treatment | Retrieving to remote afterloader | |
| 2.5 | 13.7 | 7.0 |
| 5.0 | 20.5 | |
In shorter dwell times, the transit time is relatively longer than the dwelling, and it is expected to increase the dosimetric impact of transit dose. Similarly, higher strength of 192Ir results in shorter dwell times, and the impact of transit dose is expected to increase [14, 15]. Since the strength of 192Ir is the highest immediately after the source is exchanged, the maximum source strength, i.e., So (41,401 cGy cm2 h–¹), was used for all treatment plans in this study.
Dose-volume histogram analysis
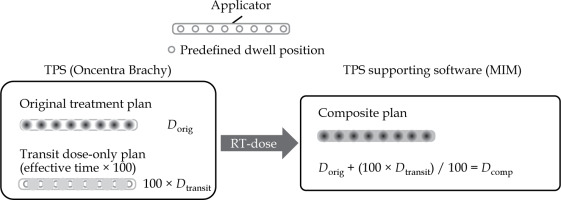
Figure 3 shows the workflow of dose-volume histogram (DVH) parameter analysis. First, dose distribution considering only the transit dose, Dtransit, was generated by setting the source at inter-dwell positions. As the effective transit time was too short and the input digits for dwell times less than 0.01 s were also not allowed by our TPS, the effective transit time multiplied by 100 was input for calculation. After this process, the calculation was multiplied by 1/100 in MIM Maestro®, version 7.1.4 (MIM Software Inc., Cleveland, OH, USA). Moreover, the original dose distribution, Dorig, and Dtrainst were summed to generate the dose distribution of composite treatment plan considering the transit dose, Dcomp, in MIM.
Fig. 3
Workflow for analyzing dose-volume histogram (DVH) parameters for treatment plans with and without transit doses
Dorig – original dose distribution, Dtransit – dose distribution only considering the transit dose, Dcomp – dose distribution of the composite plan considering the transit dose
The increment Δ of DVH parameters between the original plan (Xorig) and composite plan (Xcomp) for each brachytherapy technique was analyzed using Equation (1), as follows: ,
where X indicates clinical target volume (CTV) V100% and CTV D90%, rectum D2cc and D1cc, and bladder D2 cc and D1cc, respectively. CTV was defined as the extent of suspected vaginal invasion in gynecological cancer in the case of cervical cancer or endometrial cancer with intact uterus, and the extent included seminal vesicle in prostate cancer using CT images. We investigated D2cc for the rectum and bladder, since D2cc is a common metric. Additionally, we also considered D1cc, because the purpose of this study was to clarify the dosimetric impact of transit dose, which might be more pronounced with a smaller volume. To compare the clinical impact of transit dose, Mann-Whitney’s U test was performed for GC and PInt. Furthermore, dwell times distribution for each technique was analyzed to examine the relationships between dosimetric change in CTV D90% and dwell times.
Results
Figure 4 shows Δ of DVH parameters (V100% and D90% of CTV, D2cc and D1cc of the rectum and bladder) for each brachytherapy technique. The median Δ in CTV V100% was 0.7% for PInt, although it was < 0.1% for the other four techniques (Figure 4A). As presented in Figure 4B, the medians of Δ in CTV D90% for GC, GTO, and GH were 0.2%, 0.3%, and 0.4%, respectively. Conversely, the medians of Δ in CTV D90% for GInt and PInt were 1.0% and 1.6%, respectively. As presented in Figure 4C-F, PInt exhibited a higher Δ for D2cc and D1cc of the rectum and bladder than for the other four techniques, with the median exceeding 1.0%. All DVH parameters for PInt were statistically higher than GC (p < 0.05).
Fig. 4
Dose-volume histogram (DVH) parameter Δ for each technique: A) CTV V100%, B) CTV D90%, C) rectum D2 cc, D) rectum D1cc, E) bladder D2cc, and F) bladder D1cc
GC – cylinder applicator, GTO – tandem and two-ovoid applicators, GH – hybrid technique, GInt and PInt – interstitial techniques for gynecological and prostate cancer, respectively
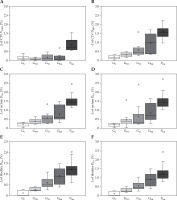
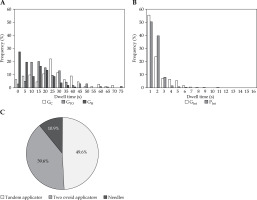
Figure 5A and B demonstrates a histogram of dwell times for each technique with S0. The median dwell times for GC, GTO, GH, GInt, and PInt were 24.1, 17.9, 8.7, 2.3, and 2.5 s, respectively. A high frequency of dwell times of less than 5 s was observed in interstitial techniques for gynecological and prostate cancers. In particular, the percentage of dwell times for each applicator type relative to total dwell times was investigated in GH (Figure 5C). As a result, a tandem, two-ovoid applicators, and needles were 49.6%, 39.6%, and 10.9%, respectively.
Fig. 5
A) Histogram of dwell times for GC (cylinder applicator), GTO (tandem and two-ovoid applicators), and GH (hybrid technique) with S0. B) Histogram of dwell times for GInt and PInt (interstitial techniques for gynecological and prostate cancers) with S0. S0 represents the highest source strength (41,401 cGy cm2 h−1). C) Percentage of dwell times for each applicator type relative to total dwell times in GH
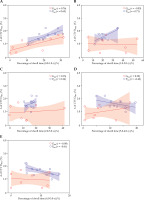
The relationship between Δ of CTV D90% and the percentage of dwell times at every second under 5 s, for GInt and PInt is illustrated in Figure 6A-E. The light-colored range in these figures represent 95% confidence interval. The percentage of dwell times less than 1.0 s correlated positively with Δ of CTV D90% for GInt and PInt, with Pearson’s correlation coefficients of 0.76 and 0.69, respectively. A similar result was obtained for PInt in the percentage of total dwell times of 1-2 s.
Fig. 6
Relationship between increment in clinical target volume (CTV) D90% for GInt and PInt, and percentage dwell times relative to total dwell times: dwell times between 0.0 and 1.0 s (A), between 1.0 and 2.0 s (B), between 2.0 and 3.0 s (C), between 3.0 and 4.0 s (D), and between 4.0 and 5.0 s (E). GInt and PInt are interstitial techniques for gynecological and prostate cancer, respectively
Discussion
In the current study, we evaluated the dosimetric impact of transit dose in clinical cases by using the effective transit time. Dissimilar to previous transit dose-related studies, which only evaluated simple conditions [7, 10, 12], our proposed straightforward method can assess the dosimetric impacts of transit dose for various brachytherapy techniques, such as a cylinder applicator, tandem and two-ovoid applicators, interstitial techniques, and hybrid technique under clinically complicated conditions, using a commercially available TPS. The comprehensively evaluated dosimetric impact observed in this study offer a novel insight that had not been proposed before.
In Figure 6, the highest correlation coefficient between Δ of CTV D90% and the percentage of dwell times are 0-1.0 s for GInt and 1.0-2.0 s for PInt. The percentage of dwell times less than 2.0 s may serve as a rough indicator for considering the transit dose, but a positive correlation was not evident for GInt and PInt when dwell times exceeded 3 s.
Fonseca et al. reported the clinical impact of an average transit dose in two interstitial techniques for prostate cases (prescribed dose at the surface of the prostate, 10 Gy) using Monte Carlo simulation (MCNP5, version 5.0) [15]. In this study, the transit dose depended on the source instantaneous speed and acceleration, and values used for source speed and strength were 300 cm s−1 and 40,700 cGy cm2 h−1, respectively. Under this condition, the authors observed that the average transit dose for two prostate cases yielded 2.3% and 1.0% increasing doses at the interest points on the surface of the prostate, respectively. Our results are consistent with those of this study, as the average increment in the mean prostate dose was 1.1% for PInt cases.
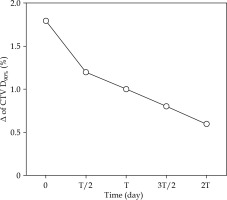
In clinical practice, the source strength is not constant; it decreases with the passing time (a half-life: T of 73.83 days). Although the evaluation of the impact of the source strength on the transit dose is clinically crucial, no previous studies have considered this factor. Figure 7 shows the change in Δ for CTV D90% over time as a function of the source strength for one patient with PInt, who had the highest Δ in CTV D90%. The zero at the lateral axes is equal to the time immediately after exchanging the source, i.e., the strength is S0. The highest Δ in CTV D90% was observed for S0, although the impact of the transit dose was less in the case of decreasing source strength. This is because longer dwell times were required to deliver the same prescribed dose, and the movement of the source was relatively shorter than that of the dwelling. Therefore, it is crucial to understand that the impact of the transit dose is larger when the source strength is high, e.g., immediately after exchanging the source.
Fig. 7
Change in Δ in clinical target volume (CTV) D90% over time as a function of source strength for one patient with PInt, who had the largest Δ in CTV D90%
T – a half-life of 192Ir (73.83 days)
This study have some limitations to consider. Indeed, the commercially available afterloader vendors have attempted to compensate the transit dose contribution for the total dose using some simple approaches [15]. For instance, in microSelectron, the delivered dwell times are decreased by about 0.1 second from the planned dwell times according to a pre-determined rule. This correction method uses some assumptions, such as brachytherapy source velocities, and it is difficult to incorporate the vendor’s correction into this study’s method. Therefore, our method assumed a situation, in which dwell times determined on TPS were directly used without any corrections for evaluation, and the transit dose increment was investigated by using the effective transit time at inter-dwell positions for various cancers and brachytherapy techniques. Although our method might overestimate the impact of the transit dose, it can be the safety side for dose evaluation. Our future research need to investigate the actual brachytherapy source movements with vender corrections by using previously described method [11] with a high-speed camera. Another limitation is that we did not account for the contribution of OAR’s dose caused by OAR-to-needle distances, since the needles might be displaced during intra-fraction, making it difficult to accurately estimate OAR’s dose. Additionally, the effective transit times for clarifying the transit doses used in this study were determined with a high-speed camera at one facility. In our previous study, the effective transit times measured with a high-speed camera demonstrated an expected uncertainty of 4 ms, owing to the limitation of its frame rate [11]. This caused an uncertainty of ~0.2% in DVH parameters, when the effective transit time was input to the commercially available TPS, exhibiting an uncertainty of 4 ms. Future studies must consider the characteristics of the source movement and the transit dose using several facilities.
Conclusions
In the present study, we proposed a new method for evaluating the dosimetric impact of the transit dose in various brachytherapy techniques, and our results indicated that the increment in DVH parameters investigated in this study by the transit dose was smaller (~0.5%) and negligible in the cylinder, tandem and two-ovoid applicators, and hybrid techniques. Conversely, the transit dose exerted a larger dosimetric impact on CTV D90% (over 1.0%) in interstitial techniques for gynecological and prostate cancers, as these methods exhibit shorter dwell times. We recommend to evaluate the impact of the transit dose when a treatment plan contains shorter dwell times less than 2 s and immediately after exchanging the source with a strong source strength.