Purpose
Prostate cancer is the most common cancer in men in developed countries, representing one-fifth of new cancer cases in the United States. In Japan, widespread prostate-specific antigen (PSA) screening revealed that prostate cancer had the highest incidence in 2016 [1, 2]. Low-dose-rate brachytherapy (LDB) using iodine-125 (125I) seeds has been approved in Japan in September 2003. Since then, it become a widely established treatment method for high-risk prostate cancer, ranging from monotherapy for low-risk localized cases to combination therapy with external beam radiation therapy or hormonal therapy. Its effectiveness and low invasiveness have made it a preferred option, including use in focal therapy in recent years [3-6]. We have previously reported on the effectiveness of LDB for prostate cancer of various risk levels, and its impact on post-operative quality of life in our hospital [7].
In brachytherapy, seed displacement during implantation is a frequent phenomenon that can disrupt operation time and dosimetry as well as increase the possibility of seed migration and dropout; therefore, it is crucial to minimize the displacement of radiation source during implantation [8, 9].
Our hospital is using Mick applicator (Mick Radio-Nuclear Instruments Inc., NY, USA), with TheraAGX100 (4.5 mm: AGX) and TheraStrand-SL (6.0 mm: TSL) radiation sources. The TSL is a radiation source, in which AGX is wrapped in a suture thread, and it is expected to improve operability, i.e., minimize seed displacement during treatment.
Research on seed displacement includes studies comparing loose seeds in cartridges and linked seeds, where multiple seeds are inserted simultaneously; however, no prospective studies have directly compared AGX and TSL regarding loose seeds [10-12].
We conducted a prospective study on the operability and treatment outcomes of AGX and TSL by evaluating the AGX and TSL seed displacement distance, seed migration/dropout count, and dose-volume histograms (DVH) of the prostate, urethra, and rectum.
Material and methods
Patients
We selected 69 patients with localized prostate cancer at the Kurume University Hospital who selected brachytherapy after receiving treatment explanations. Adverse events were observed for 2 years post-operatively.
Treatment method
An urological pathologist in our hospital confirmed histopathological diagnosis of prostate cancer from biopsy. LDB monotherapy was administered to low- and intermediate-risk patients, based on the National Comprehensive Cancer Network risk classification, with a Gleason score of 3 + 4, and a biopsy-positive core rate < 33%. The remaining intermediate-risk patients under-went additional external beam radiotherapy (EBRT) [13]. Patients in high-risk group received LDB, EBRT, and hormonal therapy from pre-treatment to 9 months post-treatment (trimodal therapy). Doses were 145 Gy for LDB monotherapy and 110 Gy for combination therapy, followed by 45 Gy of EBRT. Pathological diagnoses were made by a qualified pathologist. Treatment plan was prepared 3 weeks before LDB to confirm the prostate volume and determine the number of seeds for implantation. Neoadjuvant hormone therapy or trimodal therapy were administered to patients with prostate volumes > 40 ml at the discretion of attending urologist.
All implantations were performed using 125I loose seeds and a Mick applicator, employing interactive planning and modified peripheral loading methods. Treatment plans for all patients were generated using VariSeed version 9.0 (Varian Medical Systems, Palo Alto, CA, USA).
DVHs of the prostate, urethra, and rectum were conducted. The prostate volume received 90% of the minimum dose (D90), and 100% (V100) and 150% (V150) of the prescription dose; the urethra received 5% (UD5) and 30% (UD30) of the minimum dose; and the rectal volume received 100% (RV100) of the prescription dose. Post-implantation dose analysis was performed using computed tomography (CT) and magnetic resonance imaging (MRI) 4-5 weeks after LDB. Patients were usually discharged from the hospital 2 days after implantation. Most patients were prescribed an α-blocker (e.g., tamsulosin, silodosin, or naftopidil) or a phosphodiesterase-5 inhibitor (tadalafil); however, some patients were not prescribed any medication. Both α-blockers and phosphodiesterase-5 inhibitors were continued for at least 1 month after the plan, and subsequent medications were adjusted according to urinary symptoms. EBRT involved intensity-modulated radiation therapy with a total dose of 45 Gy in 25 fractionations, 6-8 weeks after implantation. The irradiation field covered the prostate and seminal vesicles.
Evaluation method
Data on baseline patient characteristics, treatment-related factors, and dosimetry factors were collected from medical records. Post-treatment follow-ups were performed every 3 months for the first 2 years.
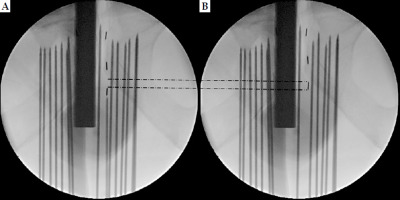
Low-dose-rate brachytherapy was conducted by a single skilled urologist (M.N.) to avoid inter-operator errors. Displacement and migration events were confirmed through ultrasound and fluoroscopic monitors by the same independent observer (N.O.). Figure 1 shows detailed images of the evaluation method. Figure 1A demonstrates the location where the radiation source was originally planned to be placed, and Figure 1B presents the radiation source displaced by one piece (1.0×) from the planned location. Displacement of radiation source was defined as displacement by 0.125 pieces (0.125×) or more. Displacement distance of radiation source was calculated from the diameter of each radiation source with AGX: 4.5 mm/piece and TSL: 6.0 mm/piece (Theragenics Corporation, United States). Pre-, intra-, and post-treatment plans, and DVH evaluations were performed by a single radiologist (C.H.). Post-treatment plan was done 1-month post-brachytherapy. A CT scan was taken on the day after brachytherapy and confirmed the course of urethra, as the catheter was still inserted. Therefore, the course of urethra at the time of post-treatment planning was estimated based on positional relationship of the seeds in CT scans taken 1 month after treatment (when preparing post-treatment plan), and on the day after brachytherapy. In previous reports, Monte Carlo investigations reported no significant differences in TG-43 values between AgX100 and TSL [14]. Therefore, a dosimetric impact from seed coating was considered negligible.
Fig. 1
Evaluation of the displacement distance of radiation source. A) Location where the radiation source was originally planned to be placed. B) Radiation source displaced by one piece (1.0×) from the planned location
The endpoints were the AGX and TSL groups’ intra- and post-treatment DVH comparisons, seed displacement incidence rate, seed displacement distance, seed migration/dropout incidence rate, and adverse events (GU – genitourinary, GI – gastrointestinal) incidence rate. Acute and late GU and GI adverse events were identified using the National Cancer Institute Common Terminology Criteria for Adverse Events version 5 (NCI CTCAE v. 5.0) [15].
Statistical analysis
Comparisons between the AGX and TSL groups, including baseline characteristics, were conducted using Student’s t test for continuous variables (Welch’s t test for displacement distance per case) and Fisher’s exact test for categorical variables. A chi-squared test was applied to compare incidence rates, such as those of adverse events. Further, a simple regression analysis was done, with operation time as the objective variable and seed type as the explanatory variable. Owing to separate purchase times of AGX and TSL, random selection was not performed (TSL was unavailable for some time). All analyses were performed using SAS v. 9.4 (SAS Institute Inc., Cary, NC, USA), and statistical significance was set at 5%. As this was a prospective observational study of consecutive cases, allocation was not applicable.
This study was approved by the Institutional Review Board of the Kurume University Hospital (approval Number: 16094), and conformed to the provisions of the Declaration of Helsinki. Informed consent was obtained from all study participants or their guardians.
Results
Patient characteristics
Table 1 shows the background information of 69 patients, with 25 cases in the AGX and 44 in the TSL groups. No significant differences were found in age, clinical T stage, Gleason score, initial PSA level, or presence/absence of hormonal therapy. A significant difference was observed in risk categories, possibly due to the inclusion of high-risk cases treated with trimodal therapy, including brachytherapy, during the TSL period. However, this difference did not influence the results of the present study, because this was an observational study of consecutive cases.
Table 1
Baseline characteristics of patients treated with AGX and TSL
Treatment outcomes
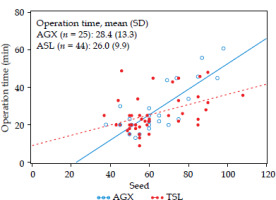
No significant differences were noted between the AGX and TSL groups regarding the treatment method, seed quantity, and needle count (Table 2). A simple regression analysis with operation time as the objective variable and seed as the explanatory variable did not show any significant difference in operation time between the two groups (AGX: 28.4 minutes vs. TSL: 26.0 minutes, p = 0.3899; Figure 2). Moreover, no significant differences were found in major treatment outcomes between DVH assessments at treatment initiation and 1-month post-treatment (Table 3). Also, there was no significant difference in the DVH over 1 month (Table 4).
Table 2
Planning phase
Table 3
Dose-volume histograms (DVH) at implant and after 1 month
Table 4
Difference in dose-volume histograms (DVH) between implant and after 1 month
Fig. 2
Seed vs. operation time
The lines represent least-square estimates from a linear regression model.
AGX: regression equation Y = –15.332 + 0.680 × X p < 0.0001
TSL: regression equation Y = 9.012 + 0.272 × X p = 0.0029
Regarding adverse events, no clear significant differences were observed between the two groups for both GU and GI adverse events (Table 5).
Table 5
Crude toxicity rate
| Grade | AGX group1 | TSL group1 | p-value2 | |
|---|---|---|---|---|
| n | 25 | 44 | ||
| GU | 0 | 9 (36.0) | 13 (29.5) | 0.7683 |
| 1 | 14 (56.0) | 25 (56.8) | ||
| 2 | 2 (8.0) | 6 (13.6) | ||
| GI | 0 | 23 (92.0) | 37 (84.1) | 0.2499 |
| 1 | 1 (4.0) | 4 (9.1) | ||
| 2 | 0 (0.0) | 3 (6.8) | ||
| 3 | 1 (4.0) | 0 (0.0) | ||
Table 6 shows the results of seed displacement in the prostate during surgery, and migration during the post-plan stage 1 month later. The values for average distance in Table 6 indicate the displacement of radiation source in terms of pieces (for example, 1.5× indicates the displacement of radiation source by 1.5 pieces). The intra-prostatic displacement incidence rate was 96.0% (24 cases) in the AGX group and 11.4% (5 cases) in the TSL group, with the TSL group having a significantly lower incidence rate. There were no significant differences in the intra-prostatic displacement count per case; however, the TSL group (average count of 1.40) tended to have fewer counts per case compared with the AGX group (average count of 4.17; p = 0.0539).
Table 6
Radiation source displacement
Additionally, no significant differences were observed in the average displacement distance. Even though the number of seeds with a displacement distance equal to at least one seed was 14 in the AGX group, no difference was observed in the TSL group (Table 6). Also, no significant differences were detected between the two groups regarding migration after 1 month or explanted dropout seed count.
Upon detailed examination of the seed displacement cases, a comparison of the displacement distance per case showed that the TSL group (2.01 mm) had a significantly shorter distance compared with the AGX group (9.22 mm) (Table 7).
Discussion
Seed displacement during surgery in brachytherapy adversely affects dosimetry, and sub-optimal seed fixity may increase the incidence of seeds dislodging into adjacent blood vessels, where they may be transported by blood flow to distant anatomical sites, such as the lungs, heart, and other organs. Therefore, reducing intra-prostatic displacement is crucial to ensure smooth treatment [16, 17]. Several studies have suggested that loose seeds enable more precise radiation source implantation than linked seeds [12, 18], but no prospective studies on intra-prostatic displacement have been conducted due to differences in radiation sources. To our knowledge, the current study is the first prospective study to demonstrate the significance of TSL in radiation source implantation by evaluating the incidence of intra-prostatic displacement in AGX and TSL groups.
In March 2016, the TheraAGX100 and TSL became available in Japan, and both radiation sources have been widely used in clinical practice. TSL is a radiation source, in which the TheraAGX100 is wrapped in polyglactin 910 thread, with major and minor diameters longer than those of AGX by 1.5 mm and 0.2 mm, respectively. TSL enables utilization of a Mick applicator, with the tip tightly bound to suture thread, and intra-prostatic displacement is expected to be reduced in TSL as compared with AGX, owing to its complex shape.
In this study, the intra-operative intra-prostatic displacement incidence rate was significantly lower in the TSL group than in the AGX group. While there were no significant differences in the intra-prostatic displacement count per case between the groups, the TSL group tended to have a lower value (AGX vs. TSL: 4.17 vs. 1.40). Notably, no instances of displacement distance equal to at least one seed were observed in the TSL group, unlike the 14 occurrences in the AGX group. Additionally, the displacement distance per case was significantly shorter in the AGX group (9.22 mm) than in the TSL group (2.01 mm), highlighting the efficacy of TSL in reducing intra-prostatic displacement. There were no significant differences in the main treatment outcomes regarding the number of seeds implanted during treatment, number of needles, DVH during treatment, and DVH for the post-plan stage 1 month later, and favorable treatment outcomes were obtained. Regarding adverse events, no significant differences were found between the two groups regarding GU, GI, or the number of migrating seeds after 1 month, and safe treatment was possible. Compared with previous reports on post-procedural seed migration, the frequencies of migration to the lungs did not differ, but we found a slightly higher rate of migration to the pelvis in our study. However, no significant difference in migration rates between the AGX and TSL groups was observed, and the incidence rates of GU and GI were equivalent to those in previous reports, suggesting that migration had little effect [9, 18]. In the ASCENDE-RT trial, the frequency of grade 2 urogenital toxicity was higher in the brachytherapy group than in the radiation therapy group (30%). In the present study, the overall rate of grade 2 urogenital toxicity was 11.6% (8/69), and there was no significant difference between the AGX and TSL groups, suggesting that the urogenital effects were slight. Furthermore, the displacement distance of AGX in this study was larger than previously reported. However, because brachytherapy using two types of radiation sources was always performed by the same operator, we believe that the surgical procedure did not affect the displacement of radiation source or toxicities between the two investigated groups [19, 20].
Previous reports have indicated that the operation time was longer with loose seeds than with linked seeds, and the operation time was expected to be shortened by improving the number of moving seeds and displacement distance in this study, but no significant differences in the operation time were observed between the two groups (AGX: 28.4 minutes vs. TSL: 26.0 minutes) [21]. The lack of a significant difference may be attributed to our facility’s very short operation time compared with that in previous reports [22]. Moreover, the short operative time may have contributed to the lack of significant differences in adverse events. The same experienced urologist and radiologist collaborated in treating all cases in our hospital, and the operation time was shorter than that in other facilities. Additionally, DVH for the post-plan stage 1 month later was expected to be better in the TSL group, yet no significant differences were observed in the main treatment outcomes. In both cases, treatment could be conducted safely and quickly for TSL surgeries.
Therapeutic interventions beyond radical therapy, including focal therapy and active surveillance, have been regarded as possible options in recent years due to low mortality rate in prostate cancer [23-25]. Focal therapy is a non-invasive treatment for clinically significant lesions in the prostate, and is selected in some cases to maintain quality of life [26-28]. Focal therapy for prostate cancer primarily consists of high-intensity focused ultrasound (HIFU), cryotherapy, and brachytherapy for localized low- and intermediate-risk cases [29, 30]. Focal therapy with brachytherapy has a favorable 5-year biochemical recurrence-free survival rate, ranging from 91.5% to 91.9% [31]. Previous studies have reported that patients in the focal therapy group demonstrated a faster post-operative recovery according to the international prostate symptom score (IPSS) and international index of erectile function (IIEF-5) than those in the overall treatment group [32]. Focal therapy using brachytherapy is a useful option for low-risk localized prostate cancer. The present study demonstrated the effectiveness of TSL for intra-prostatic displacement, and the use of TSL in focal therapy, which requires placing a radiation source in a narrower area; it may improve treatment outcomes and preserve normal tissue.
The present study has several limitations. First, the sample size at a single hospital was insufficient. Second, the observation time was limited, and an evaluation of oncological outcomes was not possible. Patients with prostate cancer can expect long-term survival, and further studies on oncological outcomes and treatment-related adverse events are necessary. Third, although this study was prospective in nature, random selection was not performed because AGX and TSL were purchased at different times. Fourth, radiation source displacement was evaluated using real-time intra-operative fluoroscopic images. In recent years, some studies have reported that the migration of placed radiation sources can be automatically detected on fluoroscopic images using a deep learning approach. This method was used in the study, and to minimize human error to the greatest extent possible, the same doctor (N.O.) evaluated and recorded radiation source displacement in real-time under fluoroscopy in all cases [33]. Despite the above-mentioned limitations, the present study supports the usefulness of TSL compared with intra-prostatic displacement. TSL may have further applications in brachytherapy, including focal therapy.
Conclusions
This prospective study suggest that TSL is safe for use. The structure wrapped in polyglactin 910 suture thread improve the seed displacement count and displacement distance compared with conventional seeds, suggesting its effectiveness against intra-prostatic displacement. Further accumulation of cases and comparative examinations of long-term treatment outcomes are necessary to validate the results of the current study.



