Purpose
According to the American Cancer Society, head and neck cancers contribute to approximately 3% of all newly diagnosed malignancies globally, with about 600,000 new cases recorded every year [1]. Early-stage treatment involves modalities, such as surgery or radiotherapy, whereas mid to late-stage treatment is predominantly comprised of radiotherapy along with systemic chemotherapy [2]. Head and neck cancers have an insidious onset, diverse pathological types, and a higher rate of development of malignancy. A few patients present with locally advanced malignancy during an initial assessment; therefore, surgical management cannot be done in such cases. Due to its’ extreme aggressiveness, nearly half of early-stage patients are highly susceptible to local and regional recurrence and lymph node metastasis after initial surgery or radiation therapy [3]. A few recent studies have demonstrated that less than 20% of patients with recurrent disease are eligible for secondary surgical treatment [4, 5]. Additionally, a few clinical patients showed an inability to tolerate surgery during an initial consultation because of advanced tumor stage, old age, poor tumor growth location, and cosmetic factors.
Stereotactic ablative brachytherapy (SABT) is clinically important brachytherapy that is effective, minimally invasive, and safe [6]. It is a stand-alone treatment for primary or recurring head and neck cancers, and can be also applied as a combination therapy with surgery, external radiotherapy, or systemic chemotherapy [7, 8]. Classic brachytherapy for head and neck tumors consist of lip, oral mucosa, mobile tongue, floor of mouth, oropharynx, nasopharynx, and paranasal sinuses. According to our previous studies, SABT is mostly applicable to patients who cannot receive external irradiation (patients who have local recurrence after previous external irradiation), and patients who refuse surgery for head and neck local lesions [9, 10]. In this technique, a radioactive source with a short range and strong penetrating power is applied in contrast to conventional radiation therapy. Therefore, once implanted into target area, it provides an uninterrupted and stable short-range irradiation, with lesser damage to surrounding normal tissues [11]. This technique is easy to operate, and provides effective radio-protection, because its’ energy decays with distance [12]. In the last few years, the utilization of 3D-printing templates (3D-PT) and computed tomography (CT)-guided technology in SABT has improved the precision for the treatment of head and neck cancers [13].
In this study, a retrospective analysis of clinical data from patients with unresectable or inoperable head and neck cancers treated with SABT was performed to analyze the efficacy and safety of SABT in treating unresectable or inoperable head and neck cancers, and to identify factors affecting its’ efficacy.
Material and methods
Patients
In this study, a retrospective analysis of outcomes of patients with unresectable or inoperable head and neck cancers treated with SABT at Affiliated Zhongshan Hospital Dalian University between October 2016 and October 2021 was conducted. The analysis included 37 cases, with 17 men (45.9%) and 20 women (54.1%), with a median age of 76 years (range, 36-95 years) and a median Karnofsky performance status (KPS) score of 80 (range, 60-90). Pathological types included 23 cases of squamous carcinoma (62.2%), five cases of papillary carcinoma in (13.5%), three cases of adenocarcinoma (8.1%), three cases of soft tissue sarcoma (8.1%), one case of basal cell carcinoma (2.7%), and two cases of the miscellaneous category (5.4%) (one case of myoepithelial carcinoma, and one case of malignant melanoma). The research protocol of Affiliated Zhongshan Hospital Dalian University was approved by ethics committees. Before receiving therapy, all patients provided informed signed consent. Clinical imaging data, pre-operative and post-operative plans, and intra-operative operational data were recorded for all patients.
Criteria for inclusion were as follows: (1) Lesions confirmed by pathology and imaging; (2) Refusal or intolerance of surgery and/or radiotherapy; (3) Appropriate puncture route: the puncture needle should avoid important nerves, bones, large blood vessels, and adjacent important organs as far as possible to reach the target area; (4) No tendency to bleed and hyper-coagulable status; (5) Tumor diameter ≤ 7 cm (considering radiation safety, clinical costs, and previous studies, we have limited the size of tumor [14]); (6) Patients in a good general condition, with a KPS ≥ 60; (7) Expected survival time of more than 3 months. Criteria for exclusion were as follows: (1) Severe bleeding tendency; (2) Severe cardio-pulmonary insufficiency; (3) Severe hepatic and renal insufficiency; (4) Acute or chronically active infection status; (5) Contraindication for anesthesia.
Pre-operative preparation and pre-planning
One week before seed implantation, patients underwent laboratory testing and imaging. Subsequently, appropriate body position was selected according to tumor location, and a vacuum pad was used to fix the posture. An enhanced CT scan was used to determine its’ location within a 3 mm thick layer. Pre-operatively, CT images were transferred to brachytherapy treatment planning system (B-TPS; Beijing Tianhang Kelin Technology Development Co., Beijing, China). The pre-operative treatment plan included an outline of gross tumor volume (GTV) and organ at risk (OAR) as per the International Commission on Radiation Units and Measurements (ICRU) No. 83 criteria [15]. Prescription dose, seed activity, coordinate template position, and direction were then established. Then, distribution, direction, and depth of the needle insertion tract were designed, number of seeds was calculated, and simulation of spatial distribution of seeds was performed. Finally, target area and OAR dose were calculated with prescribed dose of GTV D90 achieved, and OAR dose was minimized by system optimization.
Individual 3D printing template design and production
An individualized digital model of CT localization images uploaded into B-TPS, called a ‘3D printing non-co-planar template’ (3D-PNCT; Beijing Tianhang Kelin Technology Development Co., Beijing, China), and was constructed according to pre-operative planning data using 3D image processing and reverse engineering software. 3D-PNCT contained coordinate system and needle tract information. The template was then manufactured with a 3D light-curing fast prototyping machine, which contained useful information, such as tumor target area and simulated needle tract.
Intra-operative implantation
Local infiltration anesthesia with 1% lidocaine and/or combined with intravenous compound anesthesia was applied. Patients were posited appropriately as per the pre-operative positioning. Then, vacuum pads were fixed, and patients were disinfected and toweled. 3D-PNCT was precisely aligned with the body surface positioning line, laser line, and template alignment reference line on the treatment area of patient. CT scan was performed to fix the alignment between the template and tumor. The insertion needle was then punctured percutaneously to the predetermined tissue depth, along guiding hole of 3D-PNCT. A CT scan was used to monitor the insertion path and alter it according to requirement to avoid organ and vital tissue injury in the vicinity of the path. Rows of the needle were spaced 0.5~1.0 cm apart using Mick implantation gun (Radio-Nuclear Co., USA) for backward implantation of seeds (6711 type 125I radioactive seeds; Tongfu Co., China). Seeds were implanted by free-hand puncture for tumors located in the floor of the mouth, tongue, auricle of the ear, and nasal wings. Finally, a CT scan was done to observe seed distribution. Based on that, the implantation needle was increased to replenish an appropriate dose to the tumor target area, while avoiding harm to adjacent normal tissues and organs. As per the expert consensus statement of CT-guided 125I seed permanent brachytherapy [14] and the past clinical experience of our center, dose range between 110 and 160 Gy was considered safe and effective.
Post-operative evaluation and follow-up
A CT scan of patient’s target area was done three days after seed implantation. CT images were transmitted to B-TPS to verify the dose and seed dispersion. Tumor response assessments were performed three-monthly at 1, 3, and 6 months after treatment. The patients were followed up with CT or magnetic resonance imaging (MRI) scans of the head and neck every three months for the first two years, every six months for the next 2-5 years, and every twelve months thereafter. The response evaluation criteria in solid tumors (RECIST) version 1.1 was employed to assess local efficacy [16]. Adverse radiological effects after seed implantation therapy were assessed using the Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC) criteria [17].
Statistical methods
SPSS version 26.0 was applied for all statistical analyses. Kaplan-Meier method was used to assess the survival curves for local control rate (LCR) and overall survival (OS) rates, the critical values for GTV D90 were determined using receiver operating characteristic (ROC) curve, and the clinical efficacy was assessed with univariate and Cox model multifactorial methods (p < 0.05).
Results
Patients’ characteristics
In this study, 37 patients were included. Of these, 13 patients received 3D-PNCT non-assisted CT-guided radioactive seed implantation, whereas 24 received 3D-PNCT-assisted CT-guided radioactive seed implantation. The median number of implanted seeds, seed activity, GTV prescription dose, and post-operative GTV D90 was 32 (range, 3-89), 0.6 mCi (range, 0.4-0.8 mCi), 130 Gy (range, 100-160 Gy), and 127 Gy (range, 93-207 Gy), respectively. Clinical features of this cohort are demonstrated in Table 1 and Figure 1.
Table 1
Characteristics of patients with unresectable or inoperable head and neck cancers (n = 37)
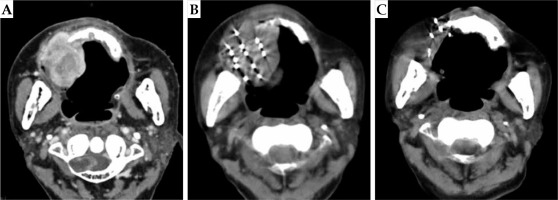
Fig. 1
A case of hypofractionated sarcoma of the right maxillary sinus. Enhanced CT of patient’s head showed space occupying lesions of the right maxillary alveolar bone and maxilla, with destruction of the skull base. Because the patient was 81 years old, the tumor had a wide range of invasion, and it was an undifferentiated sarcoma, with a high degree of malignancy. The trauma of operation was great, and the quality of life was affected after the surgery. The patient and his family were informed, and refused the operation after comprehensive consideration. Finally, the patient chose SABT. A) Pre-operative CT localization images; B) After implantation of seeds; C) Post-operative review at 18 months
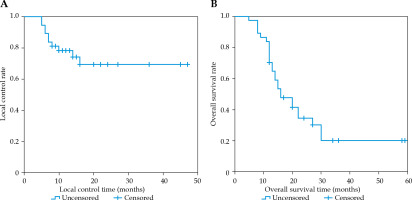
Local control effect
The median follow-up time was 34 months (range, 5-59 months). Of the 37 cases, nine responded completely, 12 cases responded partially, six patients were unstable, and 10 cases were progressive. The LCR at 6, 12, and 24 months post-operatively was 89.2%, 78.2%, and 69.4%, respectively (Figure 2). The results of univariate analysis revealed the type of pathology and GTV D90 related to LCR. The pathology of the non-squamous carcinoma group exhibited significantly better local control compared with the squamous carcinoma group (p = 0.019). Similarly, the high-dose group (GTV D90 > 117 Gy) presented higher efficacy than the low-dose group (GTV D90 ≤ 117 Gy) (p = 0.008). The type of pathology (p = 0.045) and GTV D90 (p = 0.018) were substantially related to LCR in a multivariate analysis, as shown in Figure 3.
Overall survival
The median survival time was 16 months (95% CI: 10.5-21.5 months), and the OS rate at 6, 12, and 24 months post-operatively was 97.3%, 70.3%, and 34.5%, respectively (Figure 2). The results of univariate analysis revealed that the type of pathology, GTV D90, age, and implantation site were related to the OS rate. Better survival was observed in the age group comprising of cases < 60 years compared with the age group including cases ≥ 60 years (p = 0.014). The non-squamous carcinoma group exhibited a significantly higher OS rate than the squamous carcinoma group (p < 0.001). However, the high-dose group (GTV D90 > 117 Gy) had a better OS rate than the low-dose group (GTV D90 ≤ 117 Gy) (p = 0.023). Additionally, the group with seed implantation into lymph nodes had a better OS rate than the group with primary lesions (p = 0.001). The type of pathology (p = 0.005) and GTV D90 (p = 0.016) were substantially related to the OS rate in a multivariate analysis, as demonstrated in Figure 3.
Adverse reactions
There were no complications greater than grade 3 or requiring treatment observed, including bleeding, infection, local edema, nerve injury, etc. While evaluating the post-operative radiological adverse reactions, five cases (13.5%) presented grade 1 skin reactions, two cases (5.4%) had grade 2 skin reactions, four cases (10.8%) had grade 2 oral mucosal reactions, and no other serious toxicities, such as osteo-radionecrosis of the jaw, carotid artery blowout, severe mouth opening restriction, severe dry mouth, or severe bleeding, etc. occurred. Acute reactions were relieved within six months, and no other late reaction was noted. Only in three patients with tongue cancer, post-operative seed dislocation was observed. The difference of OAR dose between patients with or without previous external irradiation was further analyzed. Statistics showed that there was a higher trend of no previous external irradiation (p < 0.05), which was related to higher dose received by these patients (Table 2).
Table 2
Summary of dose and complications with and without prior external beam radiation therapy
Discussion
For patients with head and neck cancer, who have no previous radiation history and are considered unresectable or inoperable, the primary treatment is standard chemoradiotherapy. However, some patients refuse to accept standard treatment due to basic diseases, physical causes, and other reasons. In this study, 64.9% of the patients were diagnosed with recurring head and neck cancers despite providing previous combined therapies, and were assessed by the surgeon as inoperable for repeated radical surgery. In the remaining 35.1% of the patients, no previous treatment was administered. They were confirmed as inoperable by the surgeon. A limitation of external radiotherapy is less tolerance to higher dose levels by normal tissue. For unresectable or inoperable head and neck cancer patients, providing a safe and effective treatment is a challenge. SABT has many advantages in the treatment of head and neck cancer. As a permanent implantation modality, SABT produces anti-tumor cells continuously within the target area, and easily limits the high-dose to the tumor target area. This happens due to the low radiation energy of seeds, short range, and high penetration of radiation as well as rapid dose decay to the surrounding tissues with increasing distance. Additionally, a significant reduction in radiation-related adverse reactions is observed due to the highly conformal feature [12], leading to a higher local therapeutic efficacy and lower toxic side effects.
In comparison with the overall population, older individuals with head and neck cancers have a disproportionately higher burden of comorbidities. Furthermore, head and neck cancer is frequently complicated by cerebrovascular diseases and additional non-metastatic malignancies, which worsen morbidity, mortality, and overall survival of these patients [18]. Past research performed stereotactic radiation therapy in 66 elderly patients with squamous cancer of head and neck, who were unirradiated and inoperable patients. Their one-year post-treatment LCR and OS rates were 73% and 64%, respectively, and two patients developed grade 3 toxicities [19]. Our study revealed that at one year, the LCR and OS rates after SABT treatment in age group > 60 years were 77.4% and 64.5%, respectively. Grade 3 or higher toxic reactions were not observed, indicating that SABT has similar efficacy and fewer toxic side effects compared with external radiotherapy in elderly patients. Ji et al. used 3D-printed template technology to facilitate seed implantation in post-radiation recurrent head and neck malignancies. The results indicated LCR rates at 1 and 3 years were 40.6% and 26.6%, respectively, and OS rates at 1 and 3 years were 54.3% and 15.5%, respectively. Grade 3 or higher adverse reactions were reported in 10 cases. However, no serious adverse reactions above grade 5 were observed [9]. Previous study demonstrated CT-guided 125I implants in recurrent head and neck cancer patients with an average of 20 months, and no serious adverse reactions of grade 4 or higher were noted [10]. Consistent with these findings, in this study, grade 1-2 radiological skin or oral mucosal reactions were seen in 11 patients. However, no serious adverse reactions of grade 3 or higher were observed.
In the present study, the findings of univariate analysis revealed that pathological type and GTV D90 were related to LCR. The patients with non-squamous carcinoma had better treatment outcomes compared with the squamous carcinoma patients. Additionally, the patients who received higher seed doses (GTV D90 > 117 Gy) showed better local control than patients, who received low seed doses (GTV D90 ≤ 117 Gy). The results of multivariate analysis showed that the factors affecting LCR were of pathological type and GTV D90. Of all the patients, 62.2% (23/37) had a pathological diagnosis of squamous carcinoma. The prognosis of non-squamous carcinoma was better than that of squamous carcinoma, and was partially consistent with findings of a study by Voynov et al. [20]. This was considered possible due to the following reasons. First, the pathological types of non-squamous cell carcinoma are more diverse and heterogeneous than those of squamous cell carcinoma. Especially, survival time of a few pathological types in the non-squamous cell carcinoma group, such as papillary carcinoma, adenocarcinoma, and soft tissue sarcoma, is theoretically better than that of squamous cell carcinoma. Therefore, the results of the non-squamous carcinoma treatment are superior to those of squamous carcinoma. However, this study includes incomplete types of non-squamous cell carcinoma, and an investigation of pathological types needs to be performed in the future. The reason lymph nodes are better than primary lesions is that, compared with primary tumors of head and neck, the anatomical position of lymph nodes makes the puncture easier, the shape more regular, and the conformability of dose distribution after seed implantation is better. The crucial value of GTV D90 found by ROC curve in our investigation was 117 Gy. Moreover, GTV D90 > 117 Gy had better LCR than GTV D90 ≤ 117 Gy, which is in line with previous findings [9]. This provides an important guide for planning the design and prescription of doses in SABT for unresectable and inoperable head and neck cancers. Additionally, the results of univariate analysis revealed that the type of pathology, GTV D90, age, and implantation site were related to OS rate, whereas the results of pathology and GTV D90 analysis were similar to LCR results. Furthermore, patients aged < 60 years had a better OS rate than patients aged ≥ 60 years. Patients with seeds implanted in the lymph nodes had a higher OS rate than patients with primary lesions. OS rate results in the multifactorial analyses were largely consistent with the results of local control analysis. However, due to the dose limitation to OAR, the prescription dose of some patients with previous external irradiation is limited. Considering the safety of patients and other issues, treatment should be combined with systemic therapy, such as chemotherapy, targeted therapy, etc.
SABT is safe and feasible for the treatment of unresectable and inoperable head and neck cancers. Several studies have demonstrated that SABT is appropriate for local treatment and can be combined with surgery, external irradiation, and chemotherapy to improve OS rate [21-25]. For patients with unresectable or inoperable head and neck cancers, external irradiation should be considered first, because it is non-invasive when compared with SABT. However, OAR dose of patients with previous external irradiation limits re-irradiation. The advantages of physical dosimetry and curative effect support SABT as a feasible treatment method. This single-centric retrospective study provides basic insights into the efficacy and safety of SABT in the management of unresectable or inoperable head and neck cancers using a small study sample. Therefore, further clinical evidence is required for repeated exploration and validation of the current findings. Additionally, the applicable criteria and contraindications should be improved. Nevertheless, the findings of these studies demonstrate that SABT is a safe, feasible, and minimally invasive treatment for unresectable or inoperable head and neck cancers. The high local control rate and mildly toxic side effects support the promotion and application of SABT in clinical practice.