Introduction
Ovarian cancers are “silent killers” because of their silent occurrence and slow progression [1]. Epithelial ovarian cancer is an age-related disease and is mainly a postmenopausal disease. The incidence of ovarian cancer is more pronounced in postmenopausal women above 65 years old [2].
Shen et al. found that the prevalence of epithelial ovarian cancer was significantly higher in menopausal compared to premenopausal women (42% vs. 58%) [3]. The age standard rate of ovarian cancer mortality is 3.9 [4].
The preoperative accurate diagnosis of suspected ovarian masses (OMs) is crucial to decide future therapy [5].
Malignant OMs should be managed by a gynaecology oncologist because the quality of surgical staging/lymph node dissection will subsequently affect the overall survival rate [6, 7].
Tumour markers and radiological investigations, including transvaginal sonography (TVS), have been suggested for the early detection of malignant OMs.
Multimodal screening using the CA-125 and TVS was recommended for the screening of women with suspected OMs [8, 9].
CA-125 is a tumour marker commonly used for ovarian cancer screening [10]. CA-125 is normally secreted from the ovarian epithelial and peritoneal lining cells, and cells of the gastrointestinal tract (GIT), pancreas, and lungs [11]. CA-125 is commonly elevated in epithelial ovarian tumours, breast, lung, pancreatic, and endometrial cancers, pelvic inflammatory disease (PID), endometriosis, adenomyosis, inflammatory bowel, and liver diseases [12–14].
Previous studies found that the risk of malignancy index-I (RMI-I) was an accurate tool in the primary evaluation of OMs in non-specialized gynaecology centres [1, 15].
Therefore, this study was designed to detect the accuracy of the RMI-I in diagnosing ovarian malignancy in menopausal women.
Material and methods
Eighty-two menopausal women with suspected OMs scheduled for surgery were included in this study after institutional review board approval (GN_138_19) on 13 August 2019.
Suspected OMs diagnosed in menopausal women with or without previous benign ovarian cyst and/or endometriomas were included in this study after informed consent following the Helsinki Declaration.
Exclusion criteria included women with malignant OMs under treatment, OMs with pregnancy, women with suspected or confirmed PID, or pelvic masses arising from urinary and/or GIT, and women who refused to give consent.
Women with suspected or confirmed PID were excluded from this study because PID is one of the benign causes of elevated CA-125. Elevated CA-125 in PID can subsequently increase the preoperative calculated RMI-I and the number of false-positive (FP) cases.
Pelvic inflammatory disease was suspected with the minimal diagnostic clinical criteria (lower abdominal, adnexal, and cervical motion tenderness) and confirmed by the additional diagnostic criteria (> 38.3°C, cervical mucopurulent discharge, presence of numerous white blood cells in cervicovaginal fluid, elevated erythrocyte sedimentation rate and C-reactive protein, and laboratory documentation of cervical infection with N. gonorrhoea or C. trachomatis) [16].
Participants were evaluated thoroughly and examined using the TVS to evaluate the suspected OMs regarding the consistency, whether the OMs were unilateral or bilateral, unilocular or multilocular, and the presence of extra-ovarian metastasis (using 7.5 MHz probes, Alio 400, Toshiba, Japan) by an expert sonographer (blinded to the participants’ clinical data) [17, 18]. In addition to the preoperative laboratory investigations which were done based on the hospital`s policy, blood samples were preoperatively collected from participants to measure the CA-125 [17, 18].
The risk of malignancy index-I was calculated from the CA-125 value (mIU/ml) × menopausal status × ultrasound score (CA-125 M × U) [11].
The menopausal status (M) is 1 for premenopausal women and 3 for menopausal women. Menopause is defined as > one year of amenorrhoea or > 50 years after hysterectomy (confirmed by follicle stimulating hormone level) [11].
Ultrasound score (U) scores were based on the ultrasound findings and include the consistency of the suspected OMs, whether the suspected OMs were unilateral or bilateral, unilocular or multilocular, and the presence of extra-ovarian metastasis [9]. The U score is 0 if no ultrasound findings are detected, 1 if one ultrasound finding is detected, and 3 if ≥ 2 ultrasound findings are detected within the suspected OMs [19, 20].
The excised OMs were examined histologically by an expert blinded to the participants’ clinical data, to confirm the final histological diagnosis of the excised OMs (gold standard). The preoperative calculated RMIs were compared to the postoperative histology (gold standard) of the excised OMs to detect the accuracy of RMI-I at a cut-off value of 200 in diagnosing ovarian malignancy (main outcome). The receiver operating characteristic (ROC) curve was also used to detect the cut-off value of RMI-I with the highest sensitivity and specificity in diagnosing ovarian malignancy in menopausal women.
Sample size
The required sample size was calculated using data from previous studies [14, 15] and G Power 3.1.9.4 for sample size calculation, setting the α-error probability at 0.05, power (1-β error probability) at 0.95%, and linear multiple regression model for statistical analysis. An effective sample size of ≥ 49 menopausal women was needed to produce a statistically acceptable figure.
Statistical analysis
Numerical variables were presented as mean ±SD, while categorical variables were presented as numbers and percentages (%). The accuracy of RMI-I at a cut-off value of 200 in diagnosing ovarian malignancy in the studied menopausal women was calculated. The receiver operating characteristic curve of MedCalc 20.106 (MedCalc Software Ltd., Belgium) was also used to detect the cut-off value of the RMI-I with the highest sensitivity and specificity in diagnosing ovarian malignancy in the studied menopausal women. P < 0.05 was considered significant.
This prospective study was conducted after institutional review board approval (GN_138_19) and participants’ consent following the Helsinki Declaration.
Results
Eighty-two menopausal women with suspected OMs scheduled for surgery were included in this study to detect the accuracy of RMI-I in diagnosing ovarian malignancy.
Table 1 shows the studied menopausal women`s characteristics including their age, weight, body mass index, and parity. The mean CA-125 of studied menopausal women was 55.81 ±62.8 IU/ml, while the mean US score was 2.44 ±0.9, and the mean RMI-I was 156.9 ±193.6 (Table 1).
Table 1
Characteristics of studied menopausal women
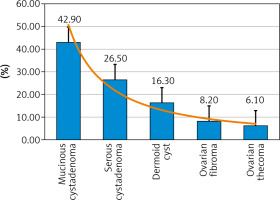
The histological examination of studied OMs (gold standard) was benign in 59.8% (49/82) and malignant in 40.2% (33/82). The commonest benign tumours in the studied menopausal women were cystadenomas (69.4%), [mucinous cystadenomas (42.9%) and serous cystadenomas (26.5%)], followed by dermoid cysts [16.3% (8/49)], ovarian fibromas [8.2% (4/49)], and ovarian thecomas [6.1% (3/49)] (Fig. 1, Table 1).
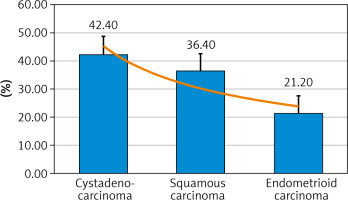
The commonest malignant tumours in the studied menopausal women were cystadenocarcinoma [42.4% (14/33)], followed by squamous carcinoma [36.4% (12/33)], and endometrioid carcinoma [21.2% (7/33)] (Fig. 2, Table 1).
Risk of malignancy index-I accuracy
The risk of malignancy index-I at cut-off value 200 was true positive (TP) in 25 cases and false negative (FN) in 8 cases, while it was true negative (TN) in 45 cases and FP in 4 cases. The risk of malignancy index-I at a cut-off value of 200 in this study had 75.8% sensitivity, 91.8% specificity, 86.2% positive predictive value (PPV), and 84.9% negative predictive value (NPV) in diagnosing ovarian malignancy in menopausal women (Table 2).
Table 2
Accuracy of the risk of malignancy index-I at a cut-off value of 200 in diagnosing ovarian malignancy in the studied menopausal women
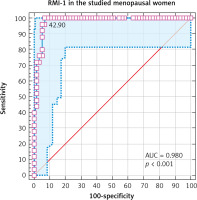
Receiver operating characteristic curve showed that the RMI-I at a cut-off value > 241.5 had 96% sensitivity and 94.74% specificity in diagnosing ovarian malignancy in menopausal women (AUC 0.98, 95% CI: 0.92–0.99, p < 0.001) (Fig. 3).
Discussion
The preoperative accurate diagnosis of suspected OMs is crucial to decide future therapy [5]. Therefore, 82 menopausal women with suspected OMs scheduled for surgery were included in this study to detect the accuracy of the RMI-I in diagnosing ovarian malignancy in menopausal women.
The histological examination of studied OMs (gold standard) was benign in 59.8% (49/82) and malignant in 40.2% (33/82).
Mythily et al. found that 88.8% of malignant OMs were diagnosed in menopausal women [14], and Jung et al. found that epithelial and sex-cord malignant OMs were more frequent in menopausal women [21].
The commonest benign tumours in the studied menopausal women were cystadenomas (69.4%), [mucinous cystadenomas (42.9%) and serous cystadenomas (26.5%)], followed by dermoid cysts [16.3% (8/49)], ovarian fibromas [8.2% (4/49)], and ovarian thecomas [6.1% (3/49)].
The commonest malignant tumours in the studied menopausal women were cystadenocarcinoma [42.4% (14/33)], followed by squamous carcinoma [36.4% (12/33)], and endometrioid carcinoma [21.2% (7/33)].
Ovarian cystadenomas are benign tumours with a good prognosis [22, 23]. The serous cystadenoma commonly occurs at the age of 40–60 years [22], while the mucinous cystadenoma is commonly diagnosed during the 3rd to 6th decades [23].
Ovarian fibromas are commonly diagnosed in premenopausal and postmenopausal women [24]. Dai et al. found that ovarian fibromas and thecomas were more common in menopausal women [25].
Al-Asadi et al. found that 52.4% of malignant OMs were cystadenocarcinomas [15], and Mythily et al. found that 60% of their studied malignant OMs were cystadenocarcinomas [14].
Dora et al. reported 32 serous and 15 mucinous cystadenocarcinomas out of 126 suspected OMs [1].
Risk of malignancy index-I
The risk of malignancy index-I at a cut-off value of 200 was suggested by many authors to diagnose ovarian cancer [26–29]. Therefore, the accuracy of RMI-I in diagnosing ovarian malignancy in menopausal women was evaluated at a cut-off value of 200, and at the best cut-off value according to the ROC curve in this study.
The risk of malignancy index-I at a cut-off value of 200 was TP in 25 cases and FN in 8 cases, while it was TN in 45 cases and FP in 4 cases. The risk of malignancy index-I at a cut-off value of 200 in this study had 75.8% sensitivity, 91.8% specificity, 86.2% PPV, and 84.9% NPV in diagnosing ovarian malignancy in menopausal women.
The receiver operating characteristic curve showed the RMI-I at a cut-off value of > 241.5 had 96% sensitivity and 94.74% specificity in diagnosing ovarian malignancy in menopausal women (AUC 0.98, 95% CI: 0.92–0.99, p < 0.001).
The difference in the RMI-I cut-off value in diagnosing ovarian malignancy depends on the prevalence of malignant OMs in the studied population [1, 30].
Dora et al. found that the RMI-I at a cut-off value 236 had 72.5% sensitivity and 98.2% specificity [1], and they concluded that the RMI-I at a cut-off value of ≥ 236 would increase the possibility of diagnosing ovarian malignancy from 54.8 to 98.15% [1].
Enakpene et al. found that the RMI-I at a cut-off value of 250 had 88.2% sensitivity and 74.3% specificity in diagnosing malignant malignancy [31].
Yamamoto et al. found the RMI-I at a cut-off value of 450 had 75% sensitivity and 91% specificity in diagnosing ovarian malignancy [32]. Adilgereyeva et al. found that the RMI-I at a cut-off value > 245.7 had 87.1% sensitivity and 100% specificity in diagnosing ovarian malignancy in menopausal women [33].
This study found that the incidence of benign and malignant OMs was 59.8% and 40.2%, respectively. The risk of malignancy index-I at a cut-off value of 200 had 75.8% sensitivity, 91.8% specificity, 86.2% PPV, and 84.9% NPV in diagnosing ovarian malignancy in menopausal women, while the ROC curve showed that the RMI-I at a cut-off value of > 241.5 had 96% sensitivity and 94.74% specificity in diagnosing ovarian malignancy in menopausal women.
The lower cut-off values increase the RMI-I sensitivity, while the higher cut-off values increase the RMI-I speci- ficity [1]. The risk of malignancy index-I cut-off value to diagnose ovarian malignancy should balance the sensitivity and specificity [34].
The current study was the first prospective study conducted to detect the accuracy of RMI-I in diagnosing ovarian malignancy in menopausal women. Some women refusing to give consent was the only limitation of this study.
Future large studies are needed to detect the most accurate RMI-I cut-off value to diagnose ovarian cancer in women with suspected OMs.
Conclusions
The risk of malignancy index-I at a cut-off value of 200 had 75.8% sensitivity, 91.8% specificity, 86.2% PPV, and 84.9% NPV in diagnosing ovarian malignancy in menopausal women. The receiver operating characteristic curve showed that the RMI-I at a cut-off value > 241.5 had 96% sensitivity and 94.74% specificity in diagnosing ovarian malignancy in menopausal women.