Purpose
The increasing incidence of non-melanoma skin cancer (NMSC) causes health problems worldwide [1]. With time and aging of the population, more and more individuals suffer from single, multiple, or advanced skin lesions, which, if not treated, may be the source of patients’ unpleasant symptoms and morbidity. The problem is complex, especially in older adults, who are often too fragile to receive intentionally curative treatment, and who frequently have multiple comorbidities and contraindications for surgery or general anesthesia. Moreover, about 5% of skin cancer patients are diagnosed with an advanced stage of the disease, showing a high loco-regional recurrence risk [2]. Equally important is their health-related quality of life (HR-QoL) that may be significantly impaired by daily problems arising from tumor-related skin disruption, inflammation, infection, function loss, exudation, or esthetic presentation [3].
Among all skin cancer cases, 80% are NMSC, of which 70% represent basal cell carcinoma (BCC) and 20% squamous cell carcinoma (SCC) [4]. In the most frequent NMSC type, BCC, clinical growth is primarily slow but consistent. It rarely forms metastases, with an incidence rate of less than 0.05% [5]. Diagnosed late, not treated, or neglected, BCC may invade subcutaneously and cause local tissue destruction leading to symptoms. Skin surgery is still listed first in the possible management options, followed by radiotherapy as a second-line primary or salvage treatment [6]. In our clinical practice, most patients are referred for brachytherapy (BT) by experienced surgeons, who assess them inoperable due to local advancement, lack of reconstructive capacity, comorbidities, contraindications to surgery or anesthesia, or disagreement for surgery. Whenever questionable, the case must be discussed in a multidisciplinary tumor board for the best treatment option. Still, multiple curative approaches may be proposed for patients over 75 years [7]. BT should be considered when hypofractionation is scheduled for patients from remote places of residence, and those with impaired mobility or tremor-related disability. Regrettably, no comparable data for managing large lesions in BT and external beam radiotherapy (EBRT) are available. Nevertheless, a meta-analysis suggests that BT may be superior to EBRT for T1-2 lesions [8]. Also, due to initial tumor significant growth and healthy tissue destruction, high BT local effectiveness may be related to possibly reduced cosmesis. However, this condition might not be an issue for elderly patients [9-11]. Aged patients are commonly underrepresented in prospective clinical trials; thus, no rigorous evidence-based data exists. Decisions are made based on clinical experience and collective case discussions, supported by published recommendations and literature reviews [10, 12-14].
The current study presented a retrospective summary of elderly patients unfit for surgery, treated with various BT techniques tailored individually for locally advanced NMSCs in a reference brachytherapy department. The findings were supported by clinical case presentations with the treatment results and sequelae.
Material and methods
Patient selection
We retrospectively identified advanced NMSCs patients older than 75 years referred to our department for treatment. All patients had pathologically confirmed skin lesions staged at least T3, according to the 8th edition of the American Joint Committee on Cancer, with tumors larger than 4 cm or invading subcutaneous tissues [15]. Each lesion was assessed clinically and measured based on a pre-plan computed tomography (CT). Due to satisfactory dense tumor tissue visibility, there were no indications for further investigation with magnetic resonance imaging (MRI) or ultrasound. Patients’ age, performance status, frailty, life expectance, disease understanding, comorbidities, symptoms, function impairment and preservation, complaints, home distance, family support, requirements, and expectations were considered. Consequently, the best suiting treatment option was chosen individually.
Treatment method
From a broad selection of type of applications, the patient was prescribed: 1) Superficial (contact, plesiotherapy) non-invasive BT based on commercially available thermoplastic mask combined with flexible plastic tubes (catheters) and Freiburg flap; 2) Interstitial (rigid needles, flexible plastic tubes); or 3) Hybrid (combination of superficial and interstitial approaches). If implanted, each patient was administered a single intravenous dose of prophylactic antibiotic (cefazolin, 1 g) up to 30 minutes before the first needle puncture. All treatment plans, except for one, were 3D plans based on CT scans from Oncentra Brachy Treatment Planning System (Elekta, Sweden). Careful delineation of highly irregular targets regarding their shapes and thicknesses was the way of their cumulative volume acquisition. If the target was thicker than 5 mm, interstitial or hybrid technique was considered for proper coverage. However, it may be more convenient for patients to use a non-invasive superficial applicator with a multilayer catheter arrangement that meets the skin surface constraints [16, 17].
According to the patients’ above-listed individual factors, the source type was chosen: 1) High-dose-rate (HDR) BT for mobile patients with ambulatory support; 2) Pulsed-dose-rate (PDR) or HDR-BT for interstitial/hybrid applications, or patients needing in-ward stay and nurse care.
Dose prescription
Currently in our clinical practice, the most frequent BT regimen for BCC lesions treated superficially is 45 Gy in 9 fractions of 5 Gy delivered every other day (three times a week, on working days); for SCC lesions, it is 45 Gy/ 9 fx. daily. We use 7 Gy/6 fx. administered twice weekly for more intense hypofractionation. In case of PDR-BT, the total dose is about 50 Gy for BCC and up to 60 Gy for SCC in hourly pulses of 0.5-0.6 Gy delivered for 4-5 consecutive days. If a patient cannot lie down for several days, we split PDR dose into applications separated by 1-2 weeks. Then, each patient’s stay takes about 24 hours, but the hourly pulse value is up to 0.9-1.0 Gy, which results in substantially longer overall treatment time (OTT).
The dose is specified to clinical target volume (CTV; gross tumor volume plus safety margin of 5-10 mm depending on tumor pathology and clinical presentation), and known, accepted recommendations are followed regarding target coverage and dose-volume constraints to organs at risk (OARs) [10, 13].
Follow-up
Generally, all our patients are invited for first control visit at four weeks after treatment completion, then after another two months, every three months up to two years, and every six months, till five years after the treatment. The most critical recorded end-points are tumor response (complete remission – CR), wound healing, early and late toxicities, symptoms release, and QoL improvement.
Results
We identified ten elderly patients with differently located locally advanced NMSCs, treated between 2007 and 2022. The mean patients’ age was 84 years (range, 75-95 years) and all cases were staged T3 with tumors larger than 4 cm (mean, 7.4 cm; range, 4.5-11.0 cm). There were six cases of BCC and four SCC. Apart from the tumor itself, the most common indication for treatment included soreness, bleeding, and exudation. Six patients were treated with HDR (iridium-192 radioactive source, nominal 10 Ci activity; MicroSelectron HDR, Nucletron/Elekta, Veenendaal, The Netherlands), and four with PDR-BT (iridium-192 radioactive source, nominal 1 Ci activity; MicroSelectron HDR, Nucletron/Elekta, Veenendaal, The Netherlands). Six patients had superficial applications, and four interstitial or hybrid applications. In implanted patients, no bacterial infections were observed. All patients completed the intended regimens, and attended control visits at least till CR was recorded. Median follow-up was 8.5 months (range, 3-35 months). Six out of ten patients died before the analysis. To our knowledge, none of the deaths were related to treated lesions. Patients and treatment characteristics are showed in Table 1.
Table 1
Patients and treatment characteristics
| Number (Figure) | Age (years) | Pathology | Anatomic location | Dimensions (cm); CTV volume (ml) | Symptoms | BT technique | Prescription, fractionation, OTT, BED, EQD2 | Follow-up (months) | Result | Life status | 3D treatment plan view |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. 1 (Fig. 1) | 83 | BCC | Thigh | 11 × 11 cm; 29.1 ml | Soreness, bleeding, exudation | Contact 3D HDR, flap | TD 50 Gy, OTT 12 days 10 × 5 Gy/daily fx. BED 75 Gy, EQD2 62.6 Gy | 35 | CR, prolonged healing | Died |  |
| No. 2 (Fig. 2) | 84 | BCC | Shoulder | 8.5 × 5 cm; 35.4 ml | Soreness, bleeding, exudation | Hybrid 3D IG PDR + flap | TD 49.5 Gy (90 pulses by 0.55 Gy/CTV every 60 minutes in 4 days), BED 57.5 Gy, EQD2 48 Gy | 12 | CR, almost healed | Alive |  |
| No. 3 (Fig. 3) | 85 | SCC | Face, most surface | N/A; 18.7 ml | Multifocal bleeding, purulent foci | Contact 3D HDR, mask | TD 45 Gy, OTT 11 days 9 × 5 Gy/daily fx. BED 67.5 Gy, EQD2 56.3 Gy | 9 | CR, healed | Died |  |
| No. 4 (Fig. 4) | 85 | BCC | Hand | 8 × 5 cm; 24.4 ml | Disfunction, rapid progression | Contact 3D HDR, mask + flap | TD 45 Gy, OTT 20 days 9 × 5 Gy/fx. every other day BED 67.5 Gy, EQD2 56.3 Gy | 7 | CR, healed | Died |  |
| No. 5 (Fig. 5) | 95 | BCC | Cheek | 4.5 × 2 cm; 3.85 ml | Soreness, bleeding | Interstitial 3D IG PDR | TD 49.5 Gy (90 pulses by 0.55 Gy/CTV every 60 minutes in 4 days) BED 57.5 Gy, EQD2 48 Gy | 4 | CR, healed | Alive |  |
| No. 6 (Fig. 6A-C) | 77 | BCC | Cheek | 4.5 × 4 cm; N/A | Soreness, inflammation, exudation | Contact 2D HDR/1 cm, flap | TD 50 Gy, OTT 12 days 10 × 5 Gy/1 cm/daily fx. BED 75 Gy, EQD2 62.6 Gy | 8 | CR, healed | Died | N/A |
| No. 7 (Fig. 6D-F) | 77 | SCC | Temple | 4.5 × 4 cm; 24.6 ml | Soreness, bleeding | Interstitial 3D IG PDR | TD 59.4 Gy (110 pulses by 0.54 Gy/CTV every 60 minutes in 5 days) BED 68.9 Gy, EQD2 57.4 Gy | 12 | CR, healed | Died |  |
| No. 8 (Fig. 6G-I) | 75 | SCC | Hand | 5 × 3 cm; 3.3 ml | Soreness, disfunction, bleeding | Contact 3D HDR, mask | TD 45 Gy, OTT 20 days 9 × 5 Gy/CTV, fx. every other day BED 67.5 Gy, EQD2 56.3 Gy | 9 | CR, healed | Alive |  |
| No. 9 (N/A) | 89 | BCC | Lower back | 10 × 6 cm; 65.6 ml | Soreness, bleeding, exudation | Hybrid 3D IG PDR + flap | TD 45 Gy/2 × 22.5 Gy (25 pulses by 0.9 Gy every 55 min.), OTT 16 days, BED 57.4 Gy, EQD2 47.8 Gy | 6 | CR, prolonged healing | Alive |  |
| No. 10 (Fig. 6J, K) | 90 | SCC | Buttock | 10 × 8 cm; 53.4 ml | Discomfort | Contact 3D HDR, mask | TD 42 Gy, OTT 18 days, 6 × 7 Gy/2 fx. per week BED 71.4 Gy, EQD2 59.5 Gy | 3 | CR, almost healed | Died |  |
[i] BCC – basal cell carcinoma, BED – biologically effective dose, BT – brachytherapy, CR – complete remission, CTV – clinical target volume, EQD2 – equivalent dose in 2 Gy fractions, fx. – fractions, FU – follow-up, HDR – high-dose-rate, IG – image-guided, OTT – overall treatment time, PDR – pulsed-dose-rate, SCC – squamous cell carcinoma, TD – total dose
Here, we presented more detailed individual clinical and treatment planning data of our patients since the literature is lacking such information. Each case data can be followed in the text, Table 1, and respective figures for complete details.
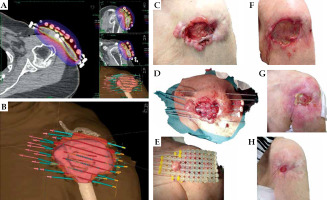
Patient No. 1 (Figure 1)
An eighty-three-year old male was referred by a skin surgeon. On the frontal surface of right thigh, he presented a diffuse BCC lesion with thickened boundaries, multiple loci of bleeding, and a vast exudating soreness inside. The lesion measured 11 × 11 cm, and was about 5-7 mm in the thickest parts (total CTV volume, 29.1 ml). The patient was proposed the simplest superficial large flap contact brachytherapy with an applicator separation of 2 cm. Due to the need for thorough wound care and lack of family support, he was admitted to our ward for the treatment course that was smoothly administered. The patient attended control visits for almost three years, presenting fast CR, and a gradual process of extensive tumor-related irradiation-induced post-lesion wound healing. For all that time, he required high-quality wound hygiene and hydrocolloid dressings. However, he expressed apparent satisfaction that the cancer was cured, and his subjective QoL (we did not provide a questionnaires) and motor skills improved.
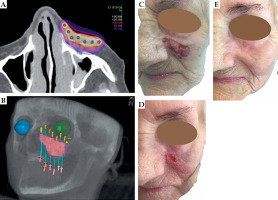
Fig. 1
Patient No. 1. Right thigh: A) Isodose cloud covering CTV; red – 100% dose, blue – 50% dose; B) 3D presentation of CTV (pink) and seven applicators (blue) inserted 2 cm apart into Freiburg flap covering the target; C) Red – 100% isodose covering CTV with visible flap shadow; D) Tumor at presentation, 11.0 × 11.0 cm; E) Tumor at the day of treatment completion; F) Five weeks after the treatment; G) Eleven months of follow-up; H) Eighteen months of follow-up; I) 2.5 years of follow-up
Patient No. 2 (Figure 2)
An eighty-four years old female presented with a large ulcerated BCC tumor on the frontal surface of her left shoulder. Centrally, the tumor disintegrated, exposing the shoulder joint and radiantly invading subcutaneous tissue, measuring 8.5 × 5.0 cm (CTV volume, 35.4 ml), with no evidence of joint or bone infiltration. The patient complained of bleeding, exudation, left arm partial function loss, and problems with sleep. She was a candidate for interstitial BT and was eligible for shallow intravenous general anesthesia. We performed a hybrid application to meet all CTV coverage requirements and referred her for a single session PDR-BT. She completed five-day-long stay inward without any problems. Almost CR was noted at the first control visit, with a few residual nodules. At three months, she achieved CR and presented clear signs of healing. Only tiny erosion was recorded at the last visit after 11 months post-treatment (Figure 2H). The patient expressed extreme satisfaction with the results, release of symptoms, and arm function improvement, and was willing to continue wound care recommendations.
Fig. 2
Patient No. 2. Left shoulder: A) Isodose cloud covering CTV; red – 100% dose, blue – 50% dose; B) 3D presentation of CTV (pink) covered by 100% isodose (red); C) Tumor at presentation, 8.5 × 5.0 cm; D) Interstitial implant in tractu: metal needles, flexible applicators, and sterile Freiburg flap elements in the wound; E) Superficial sterile adjusted Freiburg flap sutured to the skin and interstitial elements beneath; F) Four weeks after the treatment; G) Seven months of follow-up; H) Eleven months of follow-up
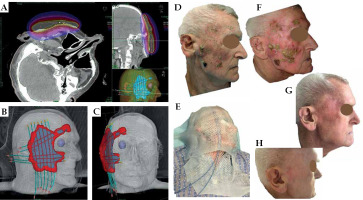
Patient No. 3 (Figure 3)
This patient was an 85-year-old man referred from a remote place of residence, in whom all possible dermatologic and surgical options were already used with no improvement. He presented with a diffuse multifocal, crusting, partially bleeding, and purulent SCC on most of his face surface. All sampled lesions were SCC. The patient was experiencing frequent itching, with the need for scratching resulting in bleeding, exudation, and dirty pillows. Due to the patient’s health status and comorbidities, we offered a stationary treatment with contact BT. An individual thermoplastic mask was prepared with multiple parallel and perpendicular bronchial applicators. A detailed 3D CT-based treatment plan was designed to cover all visible lesions completely. Five months later, the patient achieved CR with an excellent cosmetic outcome, and all irritating symptoms were released.
Fig. 3
Patient No. 3. Right side of the face: A) Isodose cloud covering CTV; red – 100% dose, blue – 50% dose; B, C) 3D presentation of CTV (blue) covered by 100% isodose (red); D) Multifocal tumor at presentation; E) Superficial thermoplastic mask placed and adjusted to the patient; F) At the day of treatment completion; G) Five months of follow-up; H) Nine months of follow-up
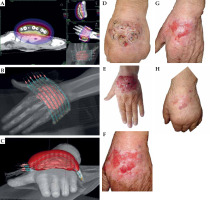
Patient No. 4 (Figure 4)
A 85-year-old male patient was referred for BT with a rapidly growing BCC of his left hand dorsum. The tumor was small and relatively stable for about five years, and later started to grow up to 8.0 × 5.0 cm, causing slight pain, itching, and hand stiffness, although it was movable and there were no signs of tendons infiltration. However, the tumor was deemed inoperable. As it is most convenient for patients, we proposed ambulatory contact BT based on a thermoplastic mask combined with a Freiburg flap and flexible catheters. We noticed an apparent increase in tumor volume in the week between the consultation and planning days. Nine doses of 5 Gy were delivered every other day, and the patient complied with the regimen very well. Six months after treatment completion, the patient presented CR with healed hand dorsum skin and complete function preservation.
Fig. 4
Patient No. 4. Left hand: A) Isodose cloud covering CTV; red – 100% dose, blue – 50% dose; B) 3D presentation of CTV (pink) covered by superficial thermoplastic mask combined with Freiburg flap and flexible applicators; C) 3D presentation of CTV (pink) covered by 100% isodose (red); D) Tumor at presentation, 8.0 × 5.0 cm; E) At the day of treatment planning (rapid growth in one week); F) Four weeks after the treatment; G) Ten weeks of follow-up; H) Six months of follow-up
Patient No. 5 (Figure 5)
A 95-year-old woman was consulted regarding treatment options for a BCC 4.5 × 2.0 cm lesion on her left cheek, invading the nasal-buccal fold and subcutaneous tissue. Despite her advanced age, she was far from being frail, with no impaired cognitive abilities. She complained of a bleeding infection, slight pain, and exudation. The consulting anesthesiologist assessed her as eligible for analgesia-based sedation; therefore, a local 2.0% xylocaine anesthesia was administered, and the patient was implanted with seven flexible interstitial tubes and prepared for a single session of PDR-BT. PDR-BT is the supreme representation of hypofractionation (understood as delivering a total dose extremely hyperfractionated into numerous pulses in a maximally short time). It enables application of a total curative dose to CTV, with minimal risk to OARs in 4-5 days. After four months of follow-up, the patient had CR, with the scar healed. She completed the treatment in March 2019, and was still alive at the manuscript’s preparation date.
Patients No. 6-10 (Figure 6)
Patient No. 6 (77-year-old female, Figure 6A-C) presented with a unique clinical case of pathologically confirmed BCC, with a deep soreness and surrounding inflammation. She refused any invasive treatment, and was proposed a contact brachytherapy. At the time of her treatment in 2007, we could offer only a Freiburg flap with plastic tubes and treatment planning on dose points (Plato System, Nucletron, Veenendaal, The Netherlands). However, she reacted perfectly to the treatment and healed with excellent cosmesis.
Patient No. 7 (77-year-old male, Figure 6D-F) had a large SCC on his temple, and was treated interstitially with PDR-BT. Again, the curative aim was achieved, and the skin under the initial lesion healed completely with a slight fibrosis.
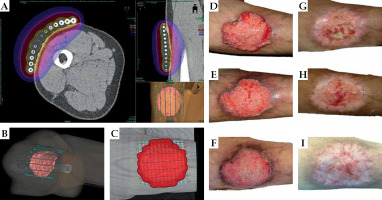
Fig. 6
Patient No. 6. Left cheek: A) Tumor at presentation, 4.5 × 4.0 cm; B) Two months after the treatment; C) Five months of follow-up. Patient No. 7. Right temple: D) Tumor at presentation, 4.5 × 4.0 cm; E) One month after the treatment; F) One year of follow-up. Patient No. 8. Right hand: G) Tumor at presentation, 5.0 × 3.0 cm; H) Six weeks after the treatment; I) Eight months of follow-up. Patient No. 10. Left buttock: J) Tumor at presentation, 10.0 × 6.0 cm; K) Four months of follow-up
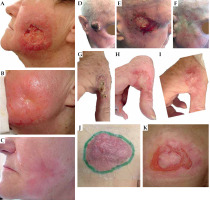
Patient No. 8 (75-year-old female, Figure 6G-I) developed SCC on her right hand. The tumor caused hand dysfunction, and a surgical proposal was a partial amputation. We prepared an individual thermoplastic mask for contact HDR-BT for unusual tumorous infiltration. The tumor dissolved, but the patient had some problems with wound healing as she was not coping with our medical advice, i.e., she was not wearing gloves for washing dishes or working in the garden. Nevertheless, repetitive instructions led to an improvement, the erosion was healed and the hand spared.
Patient No. 9 (89-year-old female, no photos) presented with a large inoperable invasive BCC on lower back, measuring 10.0 × 6.0 cm (CTV volume, 65.6 ml), showing infection, bleeding, and exuding, causing daily problems with pain and rest impairment. Due to deep infiltration, a superficial flap was supplemented by an interstitial part. The patient received PDR-BT in two separate sessions, two weeks apart. On her last follow-up, she achieved CR with prolonged post-tumoral healing.
Patient No. 10 (90-year-old male, Figure 6J, K) was referred for treatment of a large 10.0 × 8.0 cm (CTV volume, 53.4 ml) BCC on the left buttock. The tumor was a source of the patient’s discomfort and impaired resting. It was vast and relatively thick, but movable. Since the patient refused invasive treatment, a contact individual thermoplastic mask-based applicator was designed. He received 42 Gy in 6 fractions in two fractions per week on an outpatient basis. Four months later, CR was recorded, and the wound was healing very well. Although there was still an erosion, the patient reported substantial improvement of the symptoms presented before BT.
Discussion
When an oncologist consults a skin cancer patient, the first choice is surgery. A fast and radical surgical approach seems the best option. Questions arise when a patient presents with comorbidities, contraindications for invasive treatment, or challenging lesion location, and its removal can result in functional loss or poor cosmesis [18-20]. The more contraindications for surgery, the more likely the patient referral for radiotherapy, especially hypofractionated, which is proven to offer high local effectiveness with good cosmetic and functional outcomes, especially in the elderly [20-24].
Considering an individual approach and patient-adjusted clinical decisions, we often meet situations where the surgeon refuses to resect the tumor. It appears that radiation oncologists, especially interventional (brachytherapists), may offer local treatment with radioactive sources in such cases. Neglecting or hiding skin lesions from relatives and healthcare professionals may be caused by other subjective factors [25], but it is common in daily oncological practice. Our personalized approach to the presented advanced clinical examples resulted in a 100% local cure, with a median follow-up of 8.5 months, with satisfactory symptoms release and excellent cosmesis. Lancellotta et al. published similar findings of a group of 19 elderly skin cancer patients, achieving 100% CR in all radically treated patients in a median follow-up of six months. However, the group was treated only with contact thermoplastic mask-based BT [26].
Recently, patient-reported QoL issues were investigated. Despite some data suggesting that patients’ QoL may not be significantly affected, the problem exists [4]. Various treatment options and knowledge of treatment-associated factors impact older adults’ QoL and decisions. Procedures duration, post-treatment cosmesis, function, and the need for external support are all considered [27]. Nevertheless, as for BT, older age accompanied by some limitations in cooperating patients being aware of the illness, should not be a contraindication for radical treatment [28]. As there is still a lack of proper tools for skin cancer patients’ QoL assessment [29], we did not measure it objectively. However, during control visits, we received excellent subjective feedbacks from all patients being content due to cancer cure, function preservation, and good cosmesis.
After achieving CR, patients often ask if they are obliged to continue oncological visits, or if they may be referred to general practitioner (GP). Certainly, due to their health status, they were mostly referred to their GP for the best supportive care, and advised to contact an oncologist if any problem arise.
Regarding early toxicity, we assume it was mainly caused by the cancerous skin destruction (tumor-related) induced by irradiation, which uncovered underlying and surrounding healthy tissues. In such circumstances, the healing process must be naturally prolonged, and early radiation toxicity overlaps and transforms into late toxicity. Based on our observation, this phenomenon occurred in patients with tumors larger than 25 ml at presentation. During healing, the patient and family must receive detailed instructions on managing the wound properly to enhance the healing process and prevent infections, inflammations, crusting, or drying before restoring the healthy epidermis.
Occasionally, we consult more advanced skin cancer cases. For patients with simultaneously appearing multiple BCCs, with extensive and located near critical structures, we consider systemic treatment if they are not candidates for any type of radiation. Then, such patients are referred to clinical oncologists for potential treatment with hedgehog inhibitors [27, 30].
Similarly, systemic options for advanced SCCs are also available and include chemotherapy, therapy targeted on epidermal growth factor receptor (EGFR), and immunotherapy. Unfortunately, firm data and recommendations derived from controlled studies, especially for older patients, are limited. There are some advances in this field. However, the agents show adverse effects, and diverse and moderate 15-85% response rate, which is rarely durable [31-33].
We identified several weaknesses in our report. The study was retrospective in nature, presenting single institutional experience, showing results of a limited patient group; however, the patients represented a minority of the elderly with a marginal of advanced and neglected skin lesions. Furthermore, median follow-up was relatively short, and long-term survival was not considered an end-point in this selected cohort. On the other hand, we identified a strength of a broad spectrum of BT methods and techniques, enabling a very flexible and patient-oriented treatment tailoring.
Conclusions
Advanced NMSCs in elderly patients are challenging in terms of cure. Inoperable cases may be referred for feasible and locally effective interventional radiotherapy (BT). Individually tailored BT leads to an excellent disease control, function spare, symptoms release, and quality of life improvement. Large treated volumes are related to prolonged healing. BT should be discussed in a multidisciplinary tumor board regarding older patients with symptomatic functions affecting advanced NMSCs.