Purpose
Cervical cancer is one of the leading malignancies worldwide, and the fourth most frequent cancer among women in Asia [1]. Age-standardized incidence rates of cervical cancer in 2020 were 13.3 per 100,000 globally and 17.8 per 100,000 in Southeast Asia [1]. Histologically, cervical cancer can be roughly classified into squamous cell carcinoma (SCC) and adenocarcinoma. Cervical adenocarcinoma (CA) accounts for only about 20% of all cases, but its’ incidence is increasing [2-4].
Radiation therapy (RT) plays a critical role in the treatment of cervical cancer. Since the late 1990s, multiple meta-analyses have shown that concurrent chemo-RT (CCRT)/RT is the standard definitive treatment for stages IB3-IVA of the disease [2]. RT for cervical cancer comprises two important components, including external beam RT (EBRT) and brachytherapy. Recent innovations in brachytherapy have led to the use of three-dimensional image-guided brachytherapy (3D-IGBT) as the standard therapy for cervical cancer [5, 6]. Since the utilization of 3D-IGBT for cervical cancer has been recognized, many studies have shown that excellent tumor control can be achieved by administering a sufficient dose to the target [7, 8].
The concept of 3D-IGBT allowed the quantification of dose to the target, and made it possible to perform more sophisticated procedures, including interstitial brachytherapy. Interstitial approach is an advanced technique, in which multiple needles or catheters are inserted into the tumor. Although the recent National Comprehensive Cancer Network guidelines note that interstitial brachytherapy should only be performed by individuals and institutions with appropriate experience and expertise, the development of new applicators has made this approach easier to undertake [9-11]. Several reports have shown the efficacy and safety of the combination of intra-cavitary and interstitial, so-called ‘hybrid brachytherapy’ (HBT) [12, 13]. For example, a recent prospective multicenter study, EMBRACE-1, employed 3D-IGBT with interstitial technique/HBT for cervical cancer, and demonstrated that the actuarial overall 5-year local control (LC) rate was 92%, with limited severe toxicity in normal organs [14].
However, various recent studies have reported a poorer clinical outcomes of cervical adenocarcinoma or cervical adenosquamous carcinoma (CA/CAC) compared with cervical SCC, even when 3D-IGBT is applied [15, 16]. Minkoff et al. reported that CA/CAC histology was associated with inferior LC and overall survival (OS) in magnetic resonance imaging (MRI)-based 3D-IGBT [15]. Another study that employed computed tomography (CT)-based 3D-IGBT also identified CA/CAC as a poor prognostic factor for LC and OS rates [16]. These reports imply that a different treatment strategy may be needed for CA/CAC. However, the absolute number of patients with CA/CAC per institution is small, limiting the ability to analyze the disease at a single institution. Furthermore, there are very few reports on the outcomes of 3D-IGBT for CA/CAC in Asia, including Japan. Thus, we conducted a multi-institutional retrospective study on 3D-IGBT in cervical cancer in Asian countries. This study aimed to investigate the clinical outcomes in patients with CA/CAC treated with definitive RT/CCRT, with a focus on 3D-IGBT. In addition, the prognostic factors and the relationship between tumor control probability and the dose to the target volume were assessed.
Material and methods
Patients
This was a multi-institutional retrospective and observational study conducted within the framework of gynecology tumor committee of the Japanese Radiation Oncology Study Group. All institutions participating in the study can perform radical RT, including 3D-IGBT, either alone or in collaboration with other institutions. After institutional review board (IRB) approval at the primary institution (National Cancer Center Hospital, Japan; IRB Number: 2018-245), IRB approval was obtained from all participating institutions. The requirement for written informed consent was waived owing to the retrospective nature of the study. Instead, a document on the opt-out policy, through which any patient and/or their family could refuse to be included in this study, was uploaded on the webpage of each institution. The study complied with the Declaration of Helsinki.
Eligibility criteria
Patients who had undergone definitive RT/CCRT for cervical cancer between 2000 and 2016 were included in the study. The inclusion criteria were as follows: 1) Age > 20 years with previously untreated, histologically proven CA/CAC; 2) History of having undergone CT-based or MRI-based 3D-IGBT; and 3) History of having an MRI-assessed initial maximal tumor size > 4 cm before treatment. The exclusion criteria were as follows: 1) History of having received induction chemotherapy or having undergone hysterectomy or pelvic irradiation; 2) History of having received any treatment for invasive cancer within the last 5 years; 3) History of having extra-pelvic disease extension, except for 1-3 para-aortic lymph node(s) (PALN); and 4) History of having undergone palliative therapy (< 40 Gy of EBRT or received < two times of brachytherapy).
Radiation therapy
All enrolled patients underwent definitive RT comprising EBRT and 3D-IGBT, as described in our previous paper [16]. Most participating institutions in this study used 3D conformal RT with central shield (CS) in the latter part of pelvic irradiation, similar to traditional methods in Japan and some parts of Asia [17-19]. CS has been used as part of EBRT in antero-posterior/postero-anterior fields to lower the irradiation dose to the bladder and rectum. In principle, up to 45-50 Gy of radiation was delivered to the whole pelvis (WP), with a daily fraction dose of 1.8 or 2.0 Gy. After 30 or 40 Gy of WP-EBRT, a 3 or 4 cm wide CS was inserted. Then, boost EBRT of 6-10 Gy in 3-5 fractions was performed for patients with pelvic nodal metastasis. For patients with PALN metastases, 40 Gy of prophylactic EBRT to the para-aortic lymph node region was administered, followed by 16-20 Gy in 8-10 fractions of boost EBRT to the metastatic PALNs. While most institutions participating in this study used 3D conformal RT with CS in the latter part of pelvic irradiation, some used intensity-modulated radiation therapy (IMRT). In IMRT, CTV included gross tumor volume, uterine body, uterine cervix, parametrium, and upper part of the vagina. A planning target volume was created, adding adequate margins to CTV to compensate for organ motion; meanwhile, no CS-like IMRT plan was applied.
Two to six (an average of four) fractions of 3D-IGBT were administered after commencing CS irradiation or completing IMRT-based EBRT, on a weekly basis. 3D-IGBT dose varied depending on treatment strategy of each facility; however, usually 6-7 Gy was administered for high-risk clinical target volume (HR-CTV) D90. Most patients underwent CT-based 3D-IGBT [20]. In institutions where HBT was feasible, HBT was considered for the following patients: 1) Tumor extended to the pelvic wall on gynecological examination/ MRI findings prior to 3D-IGBT; 2) Tumor remained unevenly distributed on the bladder or side of the rectum; and 3) Insufficient dosing or overdose of HR-CTV in organs at risk was expected.
Data collection
Any information that would identify a patient was anonymized under the philosophy of individual respect for personality, and the collected data were securely stored and handled according to regulations of each institution and relevant government.
The following patient’s characteristics and treatment information were collected: age, the International Federation of Gynecology and Obstetrics (FIGO) stage (2018) of cancer, lymph node (N) stage of cancer, presence or absence of PALN, histological sub-types, tumor size (cm) at diagnosis and before brachytherapy as assessed by MRI, overall treatment time (days), EBRT (3D-conformal RT or IMRT), presence or absence of concurrent chemotherapy, WP irradiation dose (Gy), pelvic irradiation dose with a central CS, 3D-IGBT (typical 3D-IGBT without interstitial technique or HBT), and dosimetric parameters of 3D-IGBT (HR-CTV D90, rectum D2cc, and bladder D2cc). Cumulative EBRT and brachytherapy doses were summarized and normalized to a biological equivalent dose of 2 Gy per fraction (EQD2), using a linear-quadratic model with an α/β of 10 Gy for HR-CTV, and 3 Gy for the rectum and bladder. However, dose of pelvic irradiation with CS from the dose analyses of EQD2 for HR-CTV, rectum, and bladder was excluded, as in previous studies [17-19]. This was because it was difficult to accurately assess dose contribution to the primary tumor, rectum, and bladder in pelvic irradiation with CS [22]. Tumor reduction rate in this study was calculated using the following equation:
Overall survival, LC, progression-free survival (PFS) periods, and late toxicities assessed by the common terminology criteria for adverse events version 4.0 were collected [23]. The OS period was calculated from the start of treatment to the date when the patient was last seen, or the date of death from any cause. The progression of primary site was regarded as a local failure, and the LC period was calculated from the start of treatment to the date of local failure conformation. Primary sites included the cervix, uterine body, parametrium, and vagina for patients with initial vaginal invasion. The PFS period was calculated from the start of treatment to the date of disease progression, or death from any cause.
Statistical analyses
Overall survival, LC, and PFS rates were calculated using Kaplan-Meier method. Univariate analyses were performed using log-rank test. Factors with p < 0.05 on univariate analysis were included in multivariate analysis. Multivariate analysis was performed with Cox proportional-hazards regression model. Levene’s test was applied to examine the equality of variances for comparisons between the two groups, followed by unpaired t-test. In all tests, results were considered statistically significant at a two-sided p-value of < 0.05. Statistical package for the social sciences (SPSS) for Macintosh, version 27.0 (IBM Inc., Armonk, NY, USA) was used for all statistical analyses.
Results
Anonymized data of 498 patients from 13 institutions in Japan, one in Korea, and one in Thailand were collected for the study. A total of 36 patients met the eligibility criteria. Table 1 presents clinical characteristics of these patients. The median age was 53 (range, 34-78) years. The median follow-up periods for all patients and surviving patients were 39 (range, 9-83) and 44 (range, 15-83) months, respectively. Twenty-eight patients had adenocarcinoma, and eight had adenosquamous carcinoma. Twenty patients were positive for pelvic lymph node metastases, while six were positive for PALN metastases. At diagnosis and before brachytherapy, the median tumor sizes were 5.7 cm (range, 4.0-14.5) and 4.1 cm (range, 2.1-10.3), respectively. Twenty-nine patients received concurrent chemotherapy. All patients underwent 3D-IGBT, 15 received typical 3D-IGBT, and 21 underwent HBT.
Table 1
Patient and treatment characteristics. Summary of patient characteristics and details of treatment
[i] FIGO – International Federation of Gynecology and Obstetrics; LN – lymph node; EBRT – external beam radiotherapy; 3D-CRT – three dimensional-conformal radiation therapy; WP – whole pelvic irradiation; CS – central shielding; IMRT – intensity-modulated radiotherapy; EQD2 – equivalent dose of 2 Gy per fraction; 3D-IGBT – three-dimensional image-guided brachytherapy; HR-CTV – high-risk clinical target volume; D90 – dose to 90% of target volume; D2cc – minimum dose delivered to the highest irradiated two cc area; 1 due to the difficulty of accurate evaluation, the doses of pelvic irradiation with CS were not added to the EQD2
The 3-year OS, LC, and PFS rates for all 36 patients were 68.4% (95% confidence interval [CI]: 52.8-83.9%), 68.5% (95% CI: 53.0-83.9%), and 44.4% (95% CI: 28.2-60.7%), respectively (Figure 1). At the last follow-up, local tumor recurrence was observed in 11 of 36 patients, whose details are shown in Supplementary Table 1. Of these 11 patients, five had tumor re-growth without complete disappearance of the tumor after treatment. Furthermore, nine patients had a recurrence other than local recurrence (lymph node recurrence or distant metastasis).
Fig. 1
Kaplan-Meier survival curves of 36 patients. In each of the three Kaplan-Meier curves, red indicates overall survival (OS), blue indicates local control (LC), and green indicates progression-free survival (PFS)
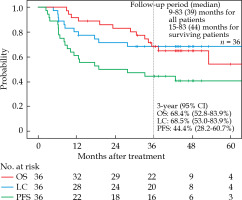
Table 2A summarizes the results of univariate analyses for each clinical factor. The tumor reduction rate and concurrent chemotherapy were found to be associated with OS. Using the median tumor reduction rate of 26.3% as the cut-off, the 3-year OS for patients with a tumor reduction rate of > 26.3% was 83.3%. The 3-year OS for patients who underwent CCRT was 78.3%, while that for patients who underwent RT alone was 28.6%. No significant differences were observed in OS in terms of FIGO stage (IB3-IIB vs. III-IVA), N stage, histological sub-types (adenocarcinoma vs. adenosquamous carcinoma), tumor size (at diagnosis or before brachytherapy), or methods of brachytherapy. The tumor reduction rate was also associated with LC or PFS. The 3-year LC and PFS rates of patients with tumor reduction rates of > 26.3% were 88.9% and 67.7%, respectively. No statistically significant differences were observed in LC or PFS in terms of other clinical factors, including FIGO stage and method of brachytherapy. Table 2B shows the results of multivariate analyses. A tumor reduction rate of > 26.3% was associated with significantly improved OS (p = 0.018), LC (p = 0.022), and PFS (p = 0.013).
Table 2
The results of univariate analyses and multivariate analysis. A) The results of univariate analyses for each clinical factor. B) The result of the assessment of prognostic factors with multivariate analyses
Regarding late rectal toxicities, four patients developed grade 1 rectal toxicity, none developed grade 2 or 3 toxicity, and one developed grade 4 rectal toxicity. Considering late bladder toxicities, two patients developed grade 2 bladder toxicity, and none developed grade 3 or worse bladder toxicity. Finally, regarding late vaginal toxicities, seven patients developed grade 1 vaginal toxicity, and one patient each developed grade 2 and grade 3 vaginal toxicities. The patient who developed grade 3 vaginal toxicity initially presented with massive tumor invasion of the vagina. For the patient who developed grade 4 rectal toxicity, rectal fistula initially presented with tumor invasion of the bladder, parametrium, and vagina. Therefore, the incidence of late adverse events of grade ≥ 3 was 5.6% (2 out of 36 patients).
The present study also assessed the relationship between dose-volume histogram parameters and oncologic outcomes (Table 3). No significant difference was found between the cumulative dose of HR-CTV D90 in brachytherapy-treated patients without and with local recurrence (mean ± standard deviation [SD]: 40.0 ±6.8 Gy EQD2, and 36.2 ±12.1 Gy EQD2, respectively; p = 0.534). The mean doses of combined brachytherapy and EBRT in patients without and with local recurrence were 76.4 ±7.5 Gy EQD2 and 71.1 ±9.1 Gy EQD2, respectively. Although the dose was slightly higher in the group without local recurrence, no statistically significant difference was observed (p = 0.294) (Table 3A).
Table 3
Relationship between dose and local control/late adverse events. A) The relationship between highrisk clinical target volume D90 dose and local control. B, C) The relationships between doses of rectum and bladder D2cc and late toxicities, respectively
Regarding the cumulative dose of rectal D2cc, the mean brachytherapy doses in patients without and with toxicity were 24.6 ±8.0 Gy EQD2 and 27.5 ±3.5 Gy EQD2, respectively. Doses of brachytherapy alone and brachytherapy plus WP in the patient with grade 4 rectal toxicity were 31.1 and 79.5 Gy EQD2, respectively, higher than the overall average. However, there were no significant differences in the mean dose of rectum D2cc between patients with and without toxicity in brachytherapy, WP, or brachytherapy plus WP (Table 3B).
Regarding the cumulative dose of bladder D2cc, the mean doses of brachytherapy in patients without and with toxicity were 38.2 ±9.8 Gy EQD2 and 41.8 ±9.4 Gy EQD2, respectively. There were no statistically significant differences in the mean dose of bladder D2cc between patients with and without toxicity in brachytherapy, WP, or brachytherapy plus WP (Table 3C).
Figure 2A shows the relationship between dose of HR-CTV D90 and tumor size at diagnosis. No specific trends in the dose of HR-CTV D90 and LC were observed in typical 3D-IGBT or HBT groups. Figure 2B demonstrates the relationship between dose of HR-CTV D90 and tumor size during brachytherapy. The mean tumor diameters in the typical 3D-IGBT and HBT groups were 3.8 ±1.2 cm and 4.7 ±1.7 cm, respectively. The tumor size was more extensive in the HBT group, although no statistically significant difference was observed (p = 0.087).
Fig. 2
Relationship between dose of HR-CTV D90 and tumor diameter in each case. A) Relationship between HR-CTV D90 dose and tumor size at diagnosis. B) Relationship between HR-CTV D90 dose and tumor size during brachytherapy
3D-IGBT – three-dimensional image-guided brachytherapy; HBT – hybrid brachytherapy; PFS – progression-free survival
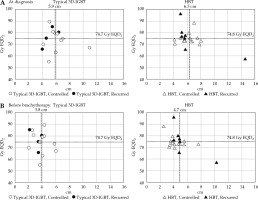
The mean dose of HR-CTV D90 of brachytherapy and EBRT in the typical 3D-IGBT and HBT groups was 74.7 ±9.4 Gy EQD2 and 74.8 ±7.6 Gy EQD2, respectively (p = 0.973). Thus, HBT could deliver a dose comparable to typical 3D-IGBT, even in large-sized tumors. However, there were no significant trends noted between LC and dose of HR-CTV D90.
Discussion
To the best of our knowledge, this is the first multi-national, multi-institutional, retrospective study to examine the significance of 3D-IGBT for CA/CAC in Asia. Many single-institutional studies have demonstrated the efficacy of 3D-IGBT for cervical cancer. The EMBRACE-1 study, a multicenter prospective cohort study, had also validated its’ efficacy [14]. In EMBRACE-1, the 5-year LC rate was 92%, and excellent LC rates of approximately 90% have been reported even in advanced stage of the disease. However, most of these studies comprised data of patients with cervical SCC; studies focusing on CA/CAC are still rare. As shown in Table 4, previous studies focused on CA/CAC used conventional 2D brachytherapy or a combination of 3D-IGBT and conventional 2D brachytherapy [24-29]. In this context, our study presents the current status of treatment using 3D-IGBT for CA/CAC. The 3-year LC rate in the present study was 68.5%. In these previous reports, the 3-year LC rates were approximately 60-70% [24-29]. OS and PFS were also far from satisfactory, probably due to the highly metastatic nature of CA/CAC [27]. Although direct comparison is inappropriate due to differences in research design and population, our results and those of previous studies suggest that 3D-IGBT does not markedly improve the LC rate of CA/CAC, and that CA/CAC has a worse prognosis than cervical SCC.
Table 4
Relevant literature and the present study. Comparison with previous studies on definitive radiation therapy for adenocarcinoma/adenosquamous carcinoma of the uterine cervix
[i] IGBT – image-guided brachytherapy; LC – local control; OS – overall survival; PFS – progression-free survival; AE – adverse event; Multi – multi-institutional study; Retro – retrospective study; N.R. – not reported; Pooled – pooled analysis; Single – single-institutional study; PSM – propensity score matching
There is no doubt that 3D-IGBT is advantageous in optimizing tumor dose while reducing the dose delivered to normal tissues. However, our results indicated that CA/CAC might not be curable even at a dose usually sufficient for treating cervical SCC. It should be noted that in comparing HR-CTV D90 doses, our study excluded the dose of pelvic irradiation with CS. Among studies that analyzed HR-CTV D90 and LC rates using the same calculation method for EQD2 as in our study, Ohno et al. reported that an LC rate > 90% was achieved in SCC > 6 cm when the total dose of WP and 3D-IGBT exceeded 66.5 Gy EQD2 [18]. Murakami et al. applied the same evaluation method, and demonstrated that the LC rate was significantly favorable in patients with HR-CTV D90 of ≥ 60 Gy EQD2 [19]. However, in the present study, local recurrence was observed in patients in whom a mean dose of 71.1 Gy EQD2 was administered. As can be seen from Figure 2, tumors recurred even when sufficient doses were administered. To date, CA/CAC has been treated using the same strategy as cervical SCC [2]. Taken together, CA/CAC may require a more individualized strategy other than the conventional strategy of increasing dose to tumor. Recently, a multi-institutional retrospective analysis of carbon-ion RT for locally advanced CA/CAC was reported [30]. With a median follow-up period of 67 months, this study showed that the 5-year LC and OS rates were 65.2% and 68.6%, respectively. These values seem to be equal to or better than those achieved using conventional treatment for CA/CAC. Furthermore, carbon-ion beams have a biological advantage owing to high linear energy transfer in the Bragg peak [31]. Therefore, carbon-ion RT may be considered as a new treatment strategy for CA/CAC. Regarding evaluation of rectal and bladder toxicities, no statistically significant differences were observed in mean doses of D2cc between patients with and without toxicity in any classification. This may be due to the small number of patients with rectal or bladder toxicities in this study. To date, a limited number of papers have discussed the relationship between late bladder and rectal toxicities and EQD2 in studies, including pelvic irradiation with CS. Thus, further studies using a larger number of patients that would clarify the dose-volume relationship for late rectal or bladder toxicities in treatment strategy, including pelvic irradiation with CS, are required.
Notably, FIGO staging did not affect LC or PFS in this study. Minkoff et al. identified the adenocarcinoma sub-type and larger GTV in 3D-IGBT as predictors of local failure [15]. Interestingly, in their study, GTV at the time of 3D-IGBT was a more potent risk factor for local recurrence, any recurrence, or death than GTV at the start of EBRT. Kusada et al. pointed out that tumor size in the transverse axis in 3D-IGBT was an independent parameter for predicting LC [17]. This suggests the need for a risk assessment of local recurrence different from conventional staging in RT for uterine CA/CAC, especially in RT involving 3D-IGBT. In this context, the results of our study that showed tumor reduction rate to be associated with LC, PFS, and OS are noteworthy. A recent multi-institutional study for carbon-ion RT for locally advanced CA/CAC also indicated that tumor shrinkage was significantly associated with LC, PFS, and OS [30]. The simple evaluation method of tumor reduction rate would be easily adaptable in actual clinical setting. In terms of analyses of tumor size reduction, Jastaniyah et al. proposed six group classifications based on the extent of tumor before treatment and its’ shrinkage [32]. In addition, Yoshida et al. morphologically classified pre-treatment tumors into two categories, such as expansive and infiltrative types, and compared the degree of shrinkage [33]. These classifications are not specific to adenocarcinoma and have not yet been analyzed in terms of clinical outcomes. Further analyses based on tumor volume may validate our findings.
The present study has several limitations, including its’ retrospective nature, various treatment regimens used, small number of patients, and time-based differences in patient care. Also, it may be insufficient in assessing treatment efficacy and toxicity over a longer period, although the median follow-up period exceeded three years. Moreover, the reason for the tumor reduction rate being associated with not only LC, but also OS and PFS, could not be clarified in the present study. A possible explanation is that the early disappearance of the tumor would reduce the risk of micro-metastasis. Another reason may be related to the immune response. Miyasaka et al. reported that patients with CA/CAC with CD8-positive tumor-infiltrating lymphocytes (CD8+ TILs) in tumor nests had significantly better OS than those without CD8+ TILs in tumor nests [28]. Although they did not mention the relationship between CD8+ TILs and LC in their study, and tumor immunogenicity may be related to LC and OS [34]. Therefore, prospective observational studies of RT/CCRT for CA/CAC, including immunogenicity evaluation, are warranted.
Conclusions
In conclusion, we reported a multi-national, multi-institutional, retrospective study examining the significance of 3D-IGBT in CA in Asia. Our results suggested that the LC rate of CA/CAC was insufficient even with 3D-IGBT. There was no clear dose-dependent response observed between the LC rate and the dose of HR-CTV D90. Meanwhile, the tumor reduction rate was associated with the LC, OS, and PFS rates. Therefore, a different treatment strategy from that applied to cervical SCC may be needed for CA/CAC.



