Introduction
Thyroid disorders are among the most common endocrine diseases. A thyroid surgery may be necessary to treat these disorders [1, 2]. Thyroidectomy is recommended for benign condition such as symptomatic large goitres and for the treatment of malignant disease of the thyroid gland [3]. Thyroidectomy has potential complications. The major postoperative complications are hypocalcaemia, wound infection, haematoma, and recurrent laryngeal nerve injury [4–6]. Hypoparathyroidism is the usual cause of hypocalcaemia and mostly occurs on the first and second days after surgery, and most physicians obtain serial serum calcium measurements after surgery to recognize and manage the low levels of calcium [7]. All patients undergoing surgery experience postoperative pain. Acute as well as chronic postoperative pain (CPOP) exists in varying degrees for every type of surgery [8]. Chronic postoperative pain is a poorly recognized potential outcome from surgery, which affects millions of patients every year, resulting in patient suffering and ensuing economic consequences. The intensity of acute postoperative pain is one of the risk factors of CPOP. Almost 37% of patients after thyroidectomy declare CPOP [9]. Parecoxib and paracetamol are non-opioid analgesics with a well-documented efficacy after different surgical procedures. The use of non-opioid analgesics can reduce opioid- induced side-effects [10, 11]. Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit the enzymes cyclooxygenase (COX)-1 and -2. Only the inhibition of COX-2 is involved in analgesic, anti-inflammatory, and antipyretic effects of NSAIDs [12]. This clinical study was designed to contrast the analgesic efficacy of 3 analgesic regimens in the setting of thyroid surgery: paracetamol monotherapy vs. paracetamol combinations with either pethidine or parecoxib. Studies investigating the analgesic effects of combined pethidine and paracetamol have not been published so far.
Material and methods
Patient selection
This prospective, randomized trial was conducted in our institution. Ethical approval was obtained from the local Ethics Committee. Between February 2017 and May 2019, 152 patients undergoing elective total or subtotal thyroidectomy were enrolled in the study. All patients provided written informed consent. Inclusion criteria were age between 35 and 65 years, American Society of Anaesthesiologists physical status classification I or II, and diagnosis of nontoxic multinodular goitre that was scheduled to be treated by elective total or subtotal thyroidectomy. Preoperative evaluation for general anaesthesia was performed. Exclusion criteria were heart failure, liver failure, renal dysfunction, diabetes, severe bronchial asthma, neurological or psychiatric disease, history of chronic pain or opioid intake, difficulty in communication due to language barriers or intellectual disability, and history of adverse events after NSAIDs (paracetamol, parecoxib) or pethidine administration. The day before surgery, the patients gave informed written consent to the study. The day prior to surgery the patients were introduced to the numerical rating scale (NRS) for pain documentation.
Study setting
All participants were randomly assigned to each group before surgery, using a computer-generated random number generator and sequentially numbered opaque sealed envelopes. Patients in group A were randomized to receive IV paracetamol 1000 mg every 8 hours and intramuscular (IM) pethidine 50 mg every 6 hours. Patients in group B were randomized to receive a combination of intravenous (IV) paracetamol 1000 mg every 8 hours and IV parecoxib 40 mg every 12 hours. Finally, patients in group C were randomized to receive IV paracetamol 1000 mg every 8 hours only. Patients who asked for more postoperative analgesics were excluded from this trial. All operations were conducted by the same group of surgeons and anaesthesiologists. General anaesthesia consisted of IV fentanyl 0.5–1.5 µg/kg and propofol. All patients received IV paracetamol 1000 mg, IV parecoxib 40 mg, and IM pethidine 50 mg during the procedure.
Postoperative pain assessment
In general, the procedure following the surgery and placement of the skin sutures is the extubation of patients in the surgical room. Surgery information is recorded, such as surgery time, and analgesics used.
Following surgery, patients were transferred to the surgical ward. Patients were evaluated at the bedside at 45 minutes, 2 hours, 6 hours, 12 hours, and 24 hours after receiving the first analgesic dose from their allocated regimen. Patients’ NRS pain ratings were recorded on postoperative monitoring charts. The scale ranges from 0 to 10, where 0 means no pain and 10 corresponds to the maximum possible pain. Four commonly used pain intensity scales are the NRS, the visual analogue scales, the verbal rating scales, and the faces pain rating scales [13–15]. There is a consensus that NRSs have more validity and more strengths than other scales [14–21]. Another reason why NRS was chosen in this trial is the fact that the medical staff and researchers are more familiar with the use of this scale.
Statistical analysis
The data were collected with the pretested questionnaires and analysed using Stata 13 statistical software. Mean and standard deviations were determined for continuous variables. The analysis of pain scores was expressed as mean and 95% confidence interval. The postoperative pain intensities measured by NRS within groups and between groups at each time interval were analysed using one-way repeat measured analysis of variance (ANOVA) and post hoc test-Bonferroni correlation. Normality of the data was tested using Shapiro-Wilk test for normality. The post hoc test was used to uncover specific differences between the 3 group means because the ANOVA test was significant. In order to use post hoc test-Bonferroni correlation, an appropriate sample size of 20 patients from each group was required for validity. P-values less than 0.05 were considered significant.
Results
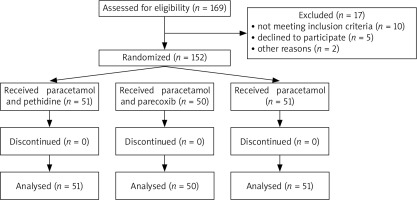
We approached 169 patients and assessed them for eligibility; 17 did not meet our inclusion criteria as shown on the flowchart in Figure 1. A total of 152 patients, including 30 males and 122 females, were randomized into 3 groups: the paracetamol and pethidine group, the paracetamol and parecoxib group, and the paracetamol (monotherapy) group. After randomization, no participant was withdrawn from the trial (Fig. 1). The mean age of the patients in the paracetamol and pethidine group (group A) was 54.1 ±3.8 years, and the mean duration time was 79.8 ±14.1 in the group. The mean ages of the patients were 52.4 ±3.5 years and 53.7 ±4.4 years for the paracetamol/parecoxib group (group B) and paracetamol monotherapy group (group C), respectively. In addition, the mean operation time for group B and group C was 81.9 ±7.4 and 80.1 ±12, respectively. The patients’ baseline characteristics are shown in Table 1.
Table 1
Baseline characteristics of the study population
Postoperative pain assessment
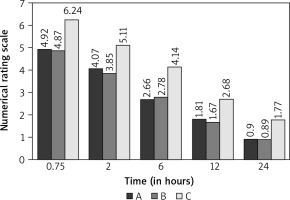
The mean NRS for patients who were treated with IV paracetamol and IM pethidine (group A) were 4.92 at 45 minutes (0.75 hours), 4.07 at 2 hours, 2.66 at 6 hours, 1.81 at 12 hours, and 0.90 at 24 hours. The mean NRS for patients who were treated with IV paracetamol and IV parecoxib (group B) were 4.87 at 45 minutes (0.75 hours), 3.85 at 2 hours, 2.78 at 6 hours, 1.67 at 12 hours, and 0.89 at 24 hours, while the mean NRS for patients who were treated with only IV paracetamol (group C) were 6.24 at 45 minutes (0.75 hours), 5.11 at 2 hours, 4.14 at 6 hours, 2.68 at 12 hours, and 1.77 at 24 hours (Fig. 2). The NRS scores of group C (paracetamol monotherapy) were significantly higher than those of groups A (pethidine + paracetamol, p < 0.001) and B (paracetamol + parecoxib, p < 0.001), while there was no significant difference between patients of group A and group B (p = 1.00).
Discussion
According to recent studies, there is no ideal postoperative analgesic treatment regimen for patients undergoing thyroid surgery. However, there are many studies that have found that nonopioid adjuncts decreased patients’ need for postoperative opioids [22]. The reduction of opioid requirements using postoperative non-opioid analgesics in patients after surgery is very important in reducing sedation, impaired pulmonary function, and constipation [23]. In our study, the influence of paracetamol and its combination with parecoxib and pethidine on postoperative consumption in a randomized, controlled trial was investigated. Patients included in this analysis underwent a thyroid surgery under general anaesthesia using a standardized surgical and anaesthetic technique. The results of this randomized, prospective study suggest that the combination of postoperative analgesic treatment with paracetamol and parecoxib is equivalent to the combination of paracetamol and pethidine. Both combinations were found to be superior to paracetamol monotherapy in achieving pain control in patients with thyroidectomy and should therefore be preferred in this setting. Furthermore, because these 2 regimens of analgesics appear to have similar efficacy, the combination of paracetamol and parecoxib should be preferred over paracetamol and pethidine, to reduce opioid consumption and associated adverse events [24, 25]. Opioid-related adverse effects in surgical patients are associated with increased length of stay in hospital and total hospital costs. The use of opioid-sparing techniques can be cost-effective [26, 27]. In a large cohort study (n = 37.031), postsurgical patients experiencing an opioid-related adverse effect had a 55% longer hospital stay, 47% higher costs, 36% increased risk of readmission, and 3.4 times higher risk of inpatient mortality [28]. In a trial in patients undergoing thyroid surgery the combination of parecoxib and paracetamol was found not to be superior to each substance alone [23]. However, studies investigating the analgesic effect of combined pethidine/paracetamol and parecoxib/paracetamol have not been published so far in patients undergoing thyroidectomy. Studies comparing the analgesic efficacy of paracetamol monotherapy vs. paracetamol combinations with either pethidine or parecoxib in patients undergoing laparoscopic cholecystectomy and open inguinal hernia repair have found the same results as our study [29, 30]. One limitation of this study that should be considered is that we did not record data during mobilization, because pain scores were recorded only at rest. The pain rating at rest alone is not very helpful because it is the functional outcome that is of clinical interest. Evaluation of pain during movement is suggested for further study [31].
Conclusions
In conclusion, the combination of postoperative analgesic treatment of IV paracetamol and IV parecoxib IV is equivalent to the combination of IV paracetamol and IM pethidine in patients undergoing thyroid surgery. Both combinations of postoperative analgesics outweigh the paracetamol monotherapy and should therefore be preferred in thyroidectomy. Furthermore, our study confirms the notion of a significant opioid-sparing effect of parecoxib in postoperative pain management after thyroidectomy.