Introduction
Autoimmune liver disorders in childhood include autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC) and de novo AIH after liver transplantation. Moreover, the AIH/PSC overlap syndrome, known as autoimmune sclerosing cholangitis (ASC), is also observed in pediatric patients with simultaneous clinical, biochemical and histologic features of both diseases. The prevalence of ASC is unknown and it is classified as a rare childhood disease [1]. The pathogenesis of autoimmune hepatobiliary disease is not fully understood, taking into account genetic and environmental factors such as viral infec- tions [2].
We present a pediatric case of ASC, which was diagnosed 2 months after severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. To the best of our knowledge, ASC potentially triggered by SARS-CoV-2 has not been noted in pediatric patients.
Case report
A 14-year-old boy was hospitalized due to fever unresponsive to antipyretic drugs, dry cough, and asthenia in the course of coronavirus disease 2019 (COVID-19) (nasopharyngeal swab turned positive in real-time polymerase chain reaction). During hospitalization, blood tests revealed increased levels of alanine aminotransferase (ALT; 292 U/l, norm: < 37 U/l) and aspartate aminotransferase (AST; 323 U/l, norm: < 40 U/l) with normal synthetic liver function. The patient was treated symptomatically without any antiviral therapy. A history of liver disease in the patient and his family has not been documented.
Two months later, due to increased activity of ALT (540 U/l) and AST (643 U/l) checked in the outpatient clinic, the patient was admitted to the tertiary gastroenterology department. At admission, the boy was completely asymptomatic and the physical examination was normal. No history of drug abuse or pharmacotherapy was disclosed; drug-induced liver injury was excluded. Laboratory tests revealed increased activity of ALT (730 U/l), AST (726 U/l), γ-glutamyl transferase (GGT; 158 U/l, norm: 9-40 U/l) and bile acid level (57.1 µmol/l, norm: 0-10 µmol/l). Serum total and direct bilirubin, albumin, lipase, C-reactive protein, creatine kinase, immunoglobulin G (IgG) levels and coagulogram were normal. Serological tests for viral hepatitis were negative (hepatitis A, B, C viruses, Epstein-Barr virus, herpes simplex virus, parvovirus B19, cytomegalovirus). Metabolic disorders such as α1-antitrypsin deficiency (serum α1-antitrypsin: 220 mg/dl, norm: 150-350 mg/dl), Wilson disease (serum ceruloplasmin 22 mg/dl, norm: 15-30 mg/dl and 24-hour urinary copper: 17 µg/24 h; norm: < 40 µg/24 h), hemochromatosis (serum ferritin 330 µg/l, norm: 30-400 µg/l), hereditary fructose intolerance (the pattern of transferrin isoforms in serum was normal), cystic fibrosis (sweat chloride value 28 mmol/l, norm: < 60 mmol/l) and celiac disease (anti-tissue transglutaminase IgA antibodies 0.1 U/ml, norm: < 10 U/ml with normal total serum IgA concentration) were also excluded. Gas chromatography-mass spectrometry (GC/MS) did not reveal urine metabolites associated with other metabolic disorders. Tests for antimitochondrial, anti-liver/kidney microsomal type 1 (anti-LKM1), anti-liver cytosol type 1 (anti-LC1) antibodies were negative and only antinuclear antibodies (ANA) were positive (1 : 320, norm: 1 : 40). During hospitalization, we did not receive the test result for anti-smooth muscle antibodies (ASMA). Abdominal ultrasound examination revealed a normal homogeneous echo texture of liver and normal spleen size. Ursodeoxycholic acid (UDCA) (15 mg/kg/day) was included in the treatment because of increased markers of cholestasis. Control tests revealed a decrease in activity of liver enzymes (ALT 624 U/l, AST 729 U/l, GGT 72 U/l). In good general condition, without any complaints, the boy was discharged home with a recommendation for a follow-up visit at the Gastrological Outpatient Clinic in 10 days.
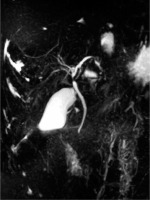
Ambulatory performed laboratory investigation confirmed increases in ALT (1084 U/l) and AST (1543 U/l) activity levels, as a result of which the boy was re-admitted to the hospital. Physical examination showed conjunctival icterus. Laboratory tests revealed increased levels of ALT (605 U/l), AST (1040 U/l), GGT (47 U/l), total bilirubin (2.55 mg/dl) and direct bilirubin (1.99 mg/dl), bile acids (364 µmol/l), as well as serum IgG (17.1 g/l), hypergammaglobulinaemia (23.9%), deranged coagulation profile (activated partial thromboplastin time 35 s, norm: 21.1-32 s, prothrombin time 15.7 s, norm: 10.4-14 s, fibrinogen 94 mg/dl, norm: 200-360 mg/dl, antithrombin 52%, norm: 80-120%) and positive ASMA (1 : 40). Ultrasonography of the abdomen showed only a small volume of pelvic free fluid. Doppler ultrasound of the hepatic vessels showed a dilated portal vein (14 mm) without other abnormalities at the level of the hepatic veins. The patient was further investigated with magnetic resonance cholangiopancreatography (MRCP), which demonstrated heterogeneous liver enhancement with an increased T2 signal predominantly in the left hepatic lobe, multifocal stricturing and dilation of intrahepatic and extrahepatic bile ducts (Fig. 1).
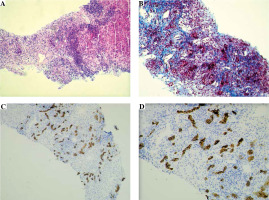
The needle liver biopsy showed a morphological picture of autoimmune liver disease with advanced fibrosis (Batts and Ludwig score 3) corresponding to the AIH/PSC overlap syndrome. Characteristic features for AIH were found, i.e. interface and lobular hepatitis, moderate/severe in nature, with intense portal and periportal lymphoplasmacytic infiltrates and severe necro-inflammatory reaction (Fig. 2A). There was advanced portal, periportal and bridging fibrosis, as well as a significant scar formation covering about half of the biopsy specimen. The process of intense fibrogenesis was accompanied by an abundant amount of mononuclear inflammatory cells in the collagen fibrous tissue (Fig. 2B). Immunohistochemical (IHC) staining of the portal tract for CK7 and CK19 revealed a proliferation of the biliary tree, which morphologically corresponds to small-duct sclerosing cholangitis [3] (Fig. 2C, D).
Fig. 2
Liver histology and immunohistochemistry of tissue material obtained from liver biopsy in a child with ASC. A) Section showing marked chronic portal and periportal intense inflammation in most portal tracts, composed of lymphocytes and plasma cells, with marked interface hepatitis (Mayer’s hematoxylin and eosin – H&E staining, original magnification 100×). B) The view of intense mononuclear infiltrates and expansion of collagen fibers around numerous, proliferating small bile ducts present within the liver scar formation, giving the morphological pattern of small-duct (autoimmune) sclerosing cholangitis (Masson’s trichrome staining, original magnification 200×). Immunohistochemical staining for CK7 well demonstrates the proliferation of numerous bile ductules within the scar formation; abundant mononuclear inflammatory cell infiltrations are present around the neoforming small ducts (IHC, original magnification: 100× (C); 200× (D))
According to the clinical, biochemical, radiological, and histological features and exclusion of different causes of elevated aminotransferases, the patient was diagnosed with ASC secondary to SARS-CoV-2 infection. We applied the ESPGHAN scoring system for juvenile autoimmune liver disease and our patient, who presented a score of 9, was identified as a definite case of ASC [1].
Treatment with prednisone (60 mg/day) was started and UDCA was continued. Later azathioprine was introduced and the steroid was progressively tapered. After two months of treatment, the patient was asymptomatic, the liver enzymes had decreased significantly (ALT 24 U/l, GGT 28 U/l), and coagulation parameters and bilirubin level were within the normal ranges. The results of the laboratory tests are summarized in Table 1.
Table 1
Laboratory results during the hospitalizations
Discussion
There are many potential causes of liver damage during SARS-CoV-2 infection, such as direct infection, use of hepatotoxic drugs, hypoxia, and the inflammatory storm [4]. The spectrum of liver involvement during COVID-19 is broad and mainly includes elevated liver enzymes and cholestasis [5]. The pathomechanism of liver damage during COVID-19 is associated with the expression of viral entry molecules, angiotensin converting enzyme 2 (ACE2), in hepatocytes and biliary epithelial cells [6]. At the moment, there are no conclusive data on the duration of elevated liver enzymes after COVID-19. Increased levels of ALT, AST and GGT were observed for several months after SARS-CoV-2 infection in adult patients [7]. Almost 75% of patients hospitalized for COVID-19 had persistently abnormal liver function tests at the last pre-discharge assessment [8]. Elevated liver enzymes during COVID-19 have also been observed in pediatric patients [5, 9]. However, data on the long-term follow-up of these patients are still lacking.
The persistently elevated level of liver enzymes is an indication for further diagnosis, taking into account viral, toxic, metabolic and autoimmune factors. Our patient underwent differential diagnosis; initially, apart from positive ANA, no other laboratory abnormalities were observed that could indicate ASC. The proteinogram and serum IgG level were normal. After a few weeks, significant increases in ALT, AST, total bilirubin, and bile acid and an abnormal coagulation profile with accompanying hypergammaglobulinemia, an increase in IgG level and positive ASMA were found. MRCP and liver biopsy confirmed a diagnosis of ASC. The presence of advanced fibrosis observed in the liver biopsy of our ASC patient suggests that the autoimmune process may have started before the COVID-19, and the infection itself accelerated the progression of the disease. The abnormal liver function parameters in our patient normalized after adding the steroid to the treatment.
Infection is a well-known factor inducing autoimmunity through an abnormal immune response triggered by epitope spreading, cross-reactions, molecular mimicry or presentation of cryptic antigens. Immune disorders such as autoimmune hemolytic anemia, immune thrombocytopenic purpura, autoimmune thyroid disease, Kawasaki disease, and Guillain-Barre syndrome that appeared secondary to COVID-19 have been described in the literature [10]. SARS-CoV-2 infection determines higher production of inflammatory cytokines such as interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), and interferon γ (IFN-γ), which may play a role in causing inflammation in the liver [1, 10].
There is scant evidence in the literature for patients presenting with ASC triggered by SARS-CoV-2 infection; therefore, the relationship between these diseases has not been established. To the best of our knowledge, autoimmune liver disease in children has not yet been described during or soon after COVID-19. There are only a few reports presenting adult AIH patients [11, 12] and only one adult case of AIH/primary biliary cholangitis overlap syndrome triggered by COVID-19 [13]. On the other hand, in patients with AIH, the course of COVID-19 was not worse than in patients with other chronic liver diseases [14, 15]. According to a multicenter study, the factors associated with higher risk of death in the AIH cohort include age, Child-Pugh class B and C cirrhosis, but not immunosuppressive therapy [15].
Conclusions
COVID-19 has been linked to a range of autoimmune diseases. Further studies are needed to determine the prevalence and quantify the clinical impact on the development of autoimmune liver disease potentially related to SARS-CoV-2 infection in pediatric patients. Our observations indicate that children with liver injury potentially caused by COVID-19 require long-term monitoring of liver function parameters.