Introduction
Anti-phospholipid antibodies (aPLs) include a very heterogeneous group of autoantibodies, directed against a variety of antigenic targets [1, 2]. Persistently elevated levels of aPLs represent important laboratory findings in the anti-phospholipid syndrome (APS), an autoimmune disease clinically manifested by vascular thrombosis and/or pregnancy complications [3-5]. According to the international classification criteria for APS anti-cardiolipin antibodies (aCLs) IgG/IgM, anti-β2-glycoprotein I antibodies (aβ2GPI) IgG/IgM or lupus anti-coagulant are involved as laboratory criteria for APS [6].
Because not all of the patients who display clinical symptoms of APS produce anti-phospholipid antibodies as listed in current APS criteria, new approaches in aPL testing have been intensively studied [7-9]. The inclusion of other aPLs into the APS criteria is considered. Anti-prothrombin, anti-phosphatidylserine/prothrombin, or aβ2GPI domain 1 antibodies are examples of antibodies, so-called non-criteria aPLs [9, 10]. However, the heterogeneity of antibodies can be expressed not only in different antigenic specificity, but also in their avidity. It is an important characteristic feature of antibodies, which describe the total binding strength between an antibody and an antigen [11]. Antibody avidities used to be examined in the antibodies directed against infectious agents, with the aim to evaluate their avidity maturation associated with the increase of specific humoral immunity [12, 13]. Avidities of autoantibodies including aPLs have already been investigated [14-19]. In contrast to anti-infectious antibodies, the autoantibody avidities can reach different values. High, low, or moderate avidity of autoantibodies occurs in autoimmune diseases. Nevertheless, it is supposed that irrespective of their avidities, autoantibodies can participate in the pathogenesis of some autoimmune diseases [20, 21].
In this pilot study, we focused on obtaining more detailed information about aCL IgG avidity. According to our knowledge, the avidity of aCLs had been explored in patients with positive aCLs, mostly in those with APS, systemic lupus erythematosus (SLE), autoimmune liver diseases, and HIV-1-infected patients [22-25]. We were interested not only in the avidity of positive aCLs, but we also analyzed if aCL avidities were connected to aCL with low levels. We paid attention to patients with low and very low levels of aCLs, considering the subjects of the 14th International Congress on Antiphospholipid Antibodies Task Force [26]. Authors of a recent study suggested that a decrease of the aPL level threshold in APS criteria could be more suitable for risk stratification of cardiovascular accident among healthy persons [27].
In the present study, we investigated the relationship between aCL IgG avidities and levels within the range of their levels, from very low to high ones, and hypothesized that very low levels of aCL might be characterized by higher avidity and that these high-avidity aCLs may be present in symptomatic patients. Moreover, we evaluated the changes of aCL levels and avidities in the group of patients during a long-term follow-up. We also examined whether the avidity of aCLs was related to levels of non-criteria types of aPLs.
Material and methods
Patients
We retrospectively analyzed 78 serum samples from 60 patients (age, 48 ±19 years; mean ±SD; sex, 36 females, 24 males). Collection of samples was conducted at the Immunological Department of the Institute of Medical Biochemistry and Laboratory Diagnostics in Prague (Czech Republic) in the period from 2011 to 2015 and in the Thomayer Hospital (Prague, Czech Republic) between 2015 and 2016. The patients were examined or hospitalized for systemic immunological diseases (n = 14, predominantly with systemic lupus erythematosus) and venous thrombosis (n = 18). The diagnosis of deep venous thrombosis was made using patients’ history, physical evaluation, and Doppler-sonography in particular. None of the studied patients had a malignant disease. The rest of the patients (n = 28) included mainly those with miscellaneous diseases, such as renal (glomerulonephritis or tubulointerstitial nephritis), gastrointestinal disorders (predominantly pancreatitis and biliary disease, such as cholelithiasis), or immunodeficiency, female infertility, and diabetes mellitus. The diagnoses were determined by experienced internists based on valid diagnostic criteria.
All subjects provided written informed consent regarding participation in the study. The ethics committees of the General University Hospital, Prague and Thomayer Hospital, Prague, have approved the study.
Methods
Levels of various types of anti-phospholipid antibodies
aCL IgG levels were determined in the sera by ELISA kits (Orgentec, Mainz, Germany). Similarly, other types of anti-phospholipid antibodies directed against other structures (anti-β2-glycoprotein I, anti-phosphatidylserine, anti-phosphatidylinositol, anti-phosphatidic acid) were also analyzed by ELISA kits (Orgentec, Mainz, Germany) in patients from the Immunological Department. According to the manufacturer recommendation, the cut-off values for aPLs were 10 GPL. Coefficients of variations for intra- and inter-assay precision of ELISAs declared by the manufacturer were mostly less than 5% and never exceeded 10%. In general, the measuring range for the aCL ELISA kit is from 0 to 120 GPL, with a functional sensitivity of 1 GPL.
Avidity of aCL IgG
The aCL IgG avidity was determined by the modified ELISA method presented by Vlachoyiannopoulos et al. [23]. In our previous study, we investigated the possibilities of aCL avidity determination using chaotropic agents in detail. We studied the most appropriate dilution of sera and concentration of urea in our modification of ELISA method for aCL avidity determination. The urea concentration of 2 mol/l and 4 mol/l did not disassociate the immune complexes sufficiently, especially if the aCL levels were higher. We found that both urea in concentrations of 6 mol/l or 8 mol/l and sodium chloride in concentrations of 1 mol/l or 2 mol/l were suitable for the sufficient dissociation of immune complexes during an extra step in the ELISA procedure. The extended description of the ELISA for avidity examination using chaotropic agents is shown in Fialová et al. [17, 18]. For the purpose of this study, we used sera diluted 1:50 and urea in concentrations of 6 mol/l and 8 mol/l as a chaotrope. The results of aCL IgG avidity were expressed as the avidity index (AI). It was calculated as a ratio (or percentage) of the residual antibodies bound in the wells in the presence of chaotropic solution (6 mol/l urea or 8 mol/l urea) to the total antibodies bound in the absence of a chaotrope.
The antibodies were classified as high-avidity ones, when the avidity index was higher than 0.6 and low-avidity ones, when the avidity index was lower than 0.4. The values of the avidity index of the antibodies from 0.4 to 0.6 were usually marked as a “grey zone” [28].
Statistics
Since the data did not express normal distribution, non-parametric statistical tests were used. The comparison of groups was performed by the Kruskal-Wallis test. The Spearman correlation coefficient was applied for correlation analyses. The paired measurements were evaluated by the Wilcoxon matched pairs test. The level of statistical significance was set as p = 0.05. Statistical analyses were performed using MedCalc (Ostend, Belgium) and Statistica programs (TIBCO Software Inc., ArcIT Consulting s.r.o., Czech Republic).
Results
Comparison of aCL IgG avidities according to aCL IgG levels
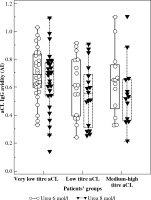
Patients were divided according to aCL IgG levels into three groups: very low level (< 10 GPL; GPL – standardized international units; n = 30), low level (10-40 GPL; n = 17), and medium-high level (> 40 GPL; n = 13). The value of aCL IgG higher than 40 GPL are designated in laboratory criteria for APS as a cut-off for medium to high levels (titers) [6, 29], and value of 10 GPL is a threshold for positive and negative outcomes declared by manufacturers for positive and negative results. If more than one sample was obtained from a patient, the serum values of the first blood sampling were evaluated. Except for one subject, no patient with thrombosis presented aCL level > 10 GPL. SLE or systemic connective tissue disease have been diagnosed in 11 patients, with aCL levels higher than 40 GPL. Figure 1 shows the values of avidity tested in the presence of urea with 6 mol/l and 8 mol/l. The avidity of aCLs did not significantly differ among groups regardless of the urea concentration used for the determination of avidity. Medians of AI were above 0.6 for all groups using urea 6 mol/l in the avidity analysis. An elevation of urea concentration for avidity determination resulted in a moderate decrease of AI medians, but no significant differences among patients’ groups were found.
Fig. 1
aCL IgG avidities in the groups of patients classified according to aCL titre (very low titre aCL < 10 GPL, n = 30; low titre aCL 10-40 GPL, n = 17; medium-high titre aCL > 40 GPL, n = 13). No significant difference was found among groups regardless of urea concentration used for avidity determination. The central box represents the values from the lower to upper quartile (25th to 75th percentile). The median is shown as the middle line. Outside and far out values are displayed as separate points.
Longitudinal evaluation of aCL IgG avidities
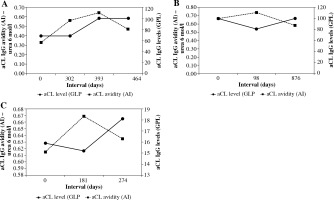
A repeated blood sampling was conducted in 14 patients in the interval of 315 days (range, 98-392 days; median 25th-75th percentile). Characteristics of longitudinally evaluated patients (age, 43 ±15 years; mean ±SD) are presented in Table 1. Paired serum samples were obtained from most patients, except for three patients, who were sampled three or four times. Patients with immunological diseases prevail in this group. The proportion of patients with low or medium-high levels of aCLs was similar among patients. The AI value of aCLs exceeded 0.6 in most patients. The aCL levels as well as their avidities did not significantly change during follow-up (Fig. 2). The time of development of levels and avidities of aCLs in three patients, who were sampled more than twice, are demonstrated in Figure 3. It is obvious that the changes in aCL levels were not uniformly accompanied by similar changes in their avidities. However, the observed changes in aCL level did not exceed the range typical for the category of aCL level, such as low or medium-high aCL levels. Similarly, the variations in aCL avidity, except for patient A, were irrelevant.
Table 1
Clinical characteristics of repeatedly examined patients
Fig. 2
Dynamics of aCL IgG titres and avidities in 14 patients during follow-up. A) Changes in aCL IgG levels; B) Changes of aCL IgG avidities using 6 mol/l urea for avidity determination; C) Changes of aCL IgG avidities using 8 mol/l urea for avidity determination. Neither aCL IgG levels, nor their avidities determined by 6 mol/l urea as well as 8 mol/l urea significantly differ during follow-up
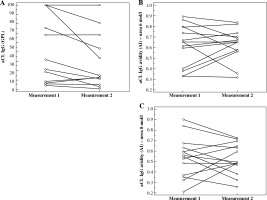
Fig. 3
Longitudinal follow-up of aCL IgG avidities and titres of in three patients. A) Patient A (female, 30 years old at the first blood sampling) was monitored for immunodeficiency; B) and C) two other patients were treated for systemic lupus erythematosus (B: male, 46 years old at the first blood sampling; C: male, 41 years old at the first blood sampling) (aCL – anti-cardiolipin antibody, AI – avidity index, GPL – standardized international units)
Correlation analyses
The aCL avidities determined using urea 6 mol/l or 8 mol/l did not correlate with their levels in any of the groups of patients classified according to aCL levels (very low, low, and medium-high aCL level groups). On the contrary, the aCL levels significantly correlated with the levels of aPLs directed against β2GPI and other phospholipids, but not with the aCL avidities (Table 2).
Table 2
Correlation between levels and avidities of aCL IgG and non-criteria aPLs. Non-criteria aPLs were analyzed in 28 patients
Discussion
In our pilot study, we investigated the relationship between avidities and aCL IgG levels across the range of their levels from very low to high ones, using the ELISA method with chaotropic agents. The aCL avidities did not differ in the groups of patients classified according to aCL levels. Higher avidity antibodies predominated in our patients, and the fluctuation of avidities in the longitudinal follow-up did not show significant differences. In addition, no relationship was found between aCL levels and avidities in our patients.
A variety of methods and their modification for antibody avidity has already been described [11, 30]. Recently, we explored in detail aCL IgG avidity methods using the principle of ELISA with chaotropes, which break the interactions of low-avidity antibodies with antigens [18]. A higher concentration of chaotropic agents caused more effective disruption of immune complexes. In this study, we applied a simple modification of the method used in the analysis of a single diluted serum exposed to a solution of urea. In our previous study [18], we found a good correlation between results obtained with a urea concentration of 6 or 8 mol/l. Nevertheless, with regards to an inclusion of serum samples with very high levels of aCLs, we performed the avidity analyses in the condition of both urea concentrations. However, independently of the used urea concentration in avidity analyses, significant differences in AI medians were not observed among tested patient groups.
Higher-avidity aCLs prevailed in our patients, since AI medians in all groups were above the value of 0.6 using urea 6 mol/l as the chaotrope. It is not surprising, because healthy persons were not included for testing. It is well-known that antibodies synthesized early in the immune response are characterized by low affinity, but the process of affinity maturation depends on many circumstances [31]. The initiation of autoimmune process is not precisely known, and many years without symptoms can precede clinical manifestation of autoimmune diseases. During this period, when the autoimmune humoral response is enhanced, levels and avidities of autoantibodies can increase [32]. The avidities of autoantibodies in autoimmune diseases studied to date have mostly reached higher values, and can participate in the severity of disease [15, 16, 33]. However, the nature of antigen seems to influence the maturation of antibody avidity. For example, avidities of anti-amyloid-b antibodies in Alzheimer’s disease were characterized by lower avidities [34]. An interesting comparison between avidities of antibodies against recall antigens and autoantigens suggested that regulation of immune B cell response can differ [35-37]. While the avidities of autoantibodies against citrullinated and carbamylated proteins, or tranglutaminase-2 tend to be low, avidities against recall antigens, such as tetanus toxoid, diphtheria toxoid, or gliadin, can be high despite their low titre. Van Delft et al. [37] explained low avidity of autoantibodies as a result of chronic antigen overload and chronic antibody responses. Unfortunately, we did not have chance to simultaneously determine avidities of both aCL and antibodies against an infectious antigen. Therefore, we cannot compare avidities of aCL with those against some recall antigen.
Suwannalai et al. [35] reported that the antibody response against autoantigens does not display avidity maturation, as observed both at baseline and at follow-up. A longitudinal examination in our study showed that the avidity of aCLs remained constant during follow-up. Our observation agrees with findings, which have been reported for several of the other autoantibodies. Cucnik et al. [14] who tested aβ2GPI avidity found no significant changes during the course of thrombotic events and pregnancy complications. Similarly, avidities of anti-citrullinated protein antibodies, anti-glutamate decarboxylase antibodies, or those against insulinoma-associated protein 2 did not exhibit extensive avidity maturation [35, 38, 39]. However, fluctuation of avidity has also been described. Avidities of anti-Hu and anti-Yo onconeural antibodies increased or remained invariable during the course of oncological diseases [40].
Only a limited number of previous studies evaluated the avidity of aCLs, eventually aβ2GPI, in the relationship to clinical manifestations are available [14, 22-24, 41-43]. Chaotropic agents such as urea or sodium chloride in ELISA have been utilized for avidity determination in these studies. Avidity of aCLs and/or aβ2GPI antibodies had been studied in patients with APS and non-APS, including those manifested as thrombosis and obstetric complications [14, 22, 23, 42]. Vlachoyiannopoulos et al. [23] found that the majority of patients with APS produced aPLs with higher avidity than non-APS patients. Predominantly, venous thrombosis was associated with higher avidity of aβ2GPI antibodies [43]. In addition, aCL avidities in patients with autoimmune liver diseases had high avidity, while the avidity of aβ2GPI in patients with HIV infection was lower in comparison with SLE/APS or primary APS patients [22, 24, 25]. However, all these studies evaluated patients with positive aPLs only.
It is known that level and avidity represent important characteristics of antibodies [44]. They are controlled by independent mechanisms. A lack of relationship between aCL IgG levels and avidities in our study does not conflict with this above-mentioned fact. Similarly, Cucnik et al. [43] observed that not all samples with high levels of aβ2GPI corresponded with high avidities.
We demonstrated a strong positive correlation between aCLs and aPLs directed against aβ2GPI and other phospholipids. Unfortunately, we had the possibility to analyze this relationship only in patients with positive aCLs. Our findings are in agreement with the conclusions of a recent systematic review stating that the prevalence of all non-criteria aPLs was significantly elevated in APS patients in comparison with those without APS [45]. Nowadays, an examination of certain non-aCL anti-phospholipid antibodies are considered mainly in clinically symptomatic, but for conventional aPLs, seronegative patients [7].
Conclusions
In summary, we are the first to evaluate aCL IgG avidity in patients not only with high levels, but also with very low ones, which are not included in the APS laboratory criteria. Our results suggested that aCL avidity did not depend on aCL levels and that high-avidity aCLs with potentially deleterious effects might also be present in patients with very low aCL levels. In addition, we confirmed that the avidity of aCLs belongs to the stable characteristics, which changes in time and are not statistically significant. In the future, we plan to extend and define our patients’ groups in greater detail and to include a group of healthy participants for further investigation of aCL avidities.


