Introduction
To date, surgery remains the only completely curative treatment for gastric cancer. Laparoscopic-assisted distal gastrectomy (LADG) has been applied extensively in the treatment of distal gastric cancer since it was first reported by Kitano [1], providing several advantages such as a small incision, minimal postoperative pain, and fast recovery [2, 3].
In recent years, with the progress of laparoscopic instruments and techniques [4, 5], totally laparoscopic distal gastrectomy (TLDG) has been increasingly adopted for gastric cancer surgery. Advantages of TLDG over LADG have been reported including less invasiveness, better cosmetic results, and a shorter hospital stay [6].
In TLDG, both lymph node dissection and digestive tract reconstruction are performed under the laparoscope, which has higher requirements for surgical skill and experience than LADG, which needs a small epigastrium auxiliary incision to perform digestive tract reconstruction extracorporeally [7].
Presently, both Billroth-II with Braun (B-IIB) reconstruction and Roux-en-Y (R-Y) reconstruction are commonly used methods to establish gastrointestinal continuity in TLDG. However, it is unclear which one of these reconstruction methods is better, as few comparative studies of B-IIB reconstruction and R-Y reconstruction in TLDG exist.
Aim
The aim of the study was to compare the efficacy of B-IIB reconstruction and R-Y reconstruction in TLDG for gastric cancer.
Material and methods
This retrospective study recruited consecutive patients who underwent TLDG for gastric cancer in our hospital from January 2019 to July 2020. The hospital’s Ethics Committee approved the study (Number: K20200812). Patients with a history of abdominal surgery, those who had undergone neoadjuvant chemotherapy, those who underwent TLDG with Billroth-I (B-I) reconstruction, and those who underwent TLDG combined with other operations were excluded. The included patients were classified into two groups according to the type of reconstruction as the B-IIB group and the R-Y group.
Variables
Clinicopathological characteristics and perioperative data were evaluated. Patient clinicopathologic characteristics included age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) status, tumor location, tumor size, pathological stage (7th American Joint Committee on Cancer) [8], and range of lymph node dissection. Perioperative data included total operative time, anastomosis time, blood loss volume, number of retrieved lymph nodes, time to first flatus, postoperative hospital stay, inflammatory parameters and complications. Postoperative complications were evaluated in accordance with the Clavien-Dindo classification [9]. Only complications of grade II or higher were regarded as events. Inflammatory parameters included white blood cell (WBC) count and C-reactive protein (CRP) level measured preoperatively and at 1 and 4 days postoperatively.
Surgical techniques
The surgical procedures were standardized. Under general anesthesia, one port below the umbilicus for laparoscopy and another four ports in the upper abdomen were used after pneumoperitoneum was instituted. After lymph node dissection performed according to the Japanese treatment guidelines for gastric carcinoma [10], the duodenum was divided close to the pyloric ring, followed by transection of the stomach. For B-IIB reconstruction, gastrojejunostomy was performed about 25 cm from the Treitz ligament in an antecolic and isoperistaltic fashion. Braun anastomosis was performed about 10 cm from the gastrojejunostomy. For R-Y reconstruction, the jejunum was divided about 15 cm from the Treitz ligament after its mesentery had been divided using ultrasonically activated shears. Gastrojejunostomy was performed in an antecolic and isoperistaltic fashion. Jejunojejunostomy was performed 40 cm distal to the gastrojejunostomy. All the intracorporeal anastomoses were completed using endoscopic staplers.
Statistical analysis
Continuous variables were presented as means ± standard deviations and analyzed by Student’s t test. Categorical variables were expressed as proportions and tested by the χ2 test or Fisher’s exact test. The p-values < 0.05 were considered statistically significant. Analyses were performed using IBM SPSS Statistics version 22.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 105 gastric cancer patients were included in the study. There were 54 patients in the B-IIB group and 51 in the R-Y group. There were no significant differences in age, sex, BMI, ASA status, tumor location, tumor size, pathological stage, and range of lymph node dissection between the two groups (p > 0.05) (Table I).
Table I
Clinicopathologic characteristics of patients
The average total operative time for the R-Y group was significantly longer than that for the B-IIB group (p < 0.001). The average anastomosis time for the R-Y group was also significantly longer than that for the B-IIB group (p < 0.001). Blood loss volume, number of retrieved lymph nodes, time to first flatus, length of postoperative hospital stay, and complications did not differ between the two groups (p > 0.05) (Table II).
Table II
Perioperative data of patients
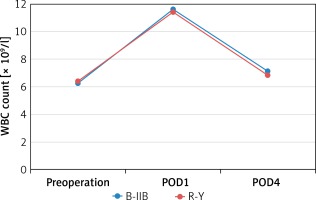
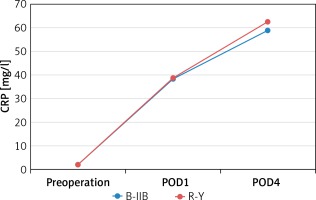
There were no significant differences between the B-IIB and R-Y groups in WBC count measured preoperatively (6.3 ±1.2 ×109/l vs. 6.4 ±1.5 × 109/l, p = 0.629) and at 1 and 4 days postoperatively (11.6 ±1.0 ×109/l vs. 11.4 ±1.0 × 109/l, p = 0.403; 7.1 ±1.0 × 109/l vs. 6.8 ±1.4 × 109/l, p = 0.210) (Figure 1). There were also no significant differences between the B-IIB and R-Y groups in CRP level measured preoperatively (2.0 ±0.6 mg/l vs. 1.9 ±0.8 mg/l, p = 0.273) and at 1 and 4 days postoperatively (38.2 ±8.6 mg/l vs. 38.8 ±10.3 mg/l, p = 0.759; 58.6 ±11.3 mg/l vs. 62.5 ±11.4 mg/l, p = 0.068) (Figure 2).
Discussion
The first surgical procedure in TLDG is to resect the distal stomach and perigastric lymph nodes radically, which accounts for primary intraoperative bleeding and embodies the oncological safety of the surgery [11]. In the present study, this procedure in the B-IIB and R-Y groups is almost identical and performed in a standardized way, which could explain the results that the number of retrieved lymph nodes and intraoperative bleeding did not differ between the study groups.
The second surgical procedure in TLDG is digestive tract reconstruction, which still remains a difficult and controversial issue [12, 13]. The ideal digestive tract reconstruction should maintain the normal anatomical and physiological functions of the digestive tract as much as possible to meet functional requirements while ensuring the safety of the anastomosis [14]. Presently, common types of digestive tract reconstruction in TLDG include B-I, B-II, and R-Y reconstructions. The gastrointestinal tract after B-I reconstruction appears anatomically and physiologically closer to the normal compared to the other two types of reconstructions, which can reduce the incidence of complications caused by gastrointestinal dysfunction [15, 16]. However, B-I reconstruction requires retaining a sufficiently long duodenum and more residual stomach to ensure that the anastomotic stoma is unrestricted [17, 18]. Therefore, this method is highly restricted by the size and location of the tumor. By contrast, B-II and R-Y reconstructions have a more adequate scope for the removal of the stomach and thus have a wider range of applications than B-I reconstruction.
However, simple B-II reconstruction has a higher incidence of complications than R-Y reconstruction such as afferent loop obstruction and intra-abdominal hernia, and the occurrence of alkaline reflux gastritis and anastomotic stomatitis is common [19]. For these reasons, Braun anastomosis can often be performed between the afferent and efferent loops after B-II anastomosis so that bile, duodenal juice, and pancreatic juice can enter directly into the efferent loop [20]. This method could help reduce the incidence of postoperative complications and improve the patients’ quality of life. Indeed, in our hospital, we routinely perform Braun anastomosis following B-II anastomosis.
In TLDG, the safety of anastomosis is of primary concern. In our study, no anastomosis-related complications occurred in the study groups. There were no significant differences in postoperative outcomes between the study groups either. These favorable results, which we attribute to the accumulation of operative experience and the use of endoscopic staplers, demonstrate that both B-IIB and R-Y reconstructions are safe to perform in TLDG. Intracorporeal anastomosis in TLDG is difficult to perform and requires a high level of experience [21–23]. All surgeons who participated in the current study had abundant prior experience in open gastrectomy and LADG, and had completed the training required for TLDG before beginning the study. All the intracorporeal anastomoses in this study were completed using endoscopic staplers, which can easily enter and exit the abdominal cavity through the trocar and help perform the anastomoses more conveniently than hand-sewing techniques.
In this study, the average total operative time and anastomosis time for the R-Y group were longer than those for the B-IIB group. This is mainly because R-Y reconstruction is more complex to perform than B-IIB reconstruction, with the additional steps of dividing the jejunum and its mesentery. For this reason, B-IIB reconstruction could be recommended as a better choice for surgeons inexperienced in TLDG.
The major limitation of the current study was its retrospective design. Also, the sample size was not large. Since the study evaluated the short-term outcomes of B-IIB and R-Y reconstructions in TLDG, future prospective studies focusing on the patients’ quality of life and long-term survival will be required to further evaluate this issue.
Conclusions
In this study, the type of reconstruction had no influence on surgical outcomes, which indicates that both B-IIB reconstruction and R-Y reconstruction are safe and effective in TLDG. B-IIB reconstruction is easier and faster to perform than R-Y reconstruction in TLDG, and therefore could be recommended as a priority for surgeons unfamiliar with this technique.