Purpose
Prostate cancer remains a major health concern in the United States, with an estimated 161,360 diagnoses in 2017 alone [1]. However, in the era of prostate-specific antigen (PSA) surveillance, a majority of men have localized disease at presentation [2,3]. In this modern era, there are many management strategies available to those with localized disease, including active surveillance, radical prostatectomy, and radiation therapy. With high survival rates associated with each of these techniques, men and their partners often make a treatment decision based on their understanding of quality of life (QOL) differences between each treatment modality [4]. There have been multiple prospective studies assessing patient-reported toxicity differences among the 3 major definitive therapy options: prostatectomy, external beam radiation therapy (EBRT), and brachytherapy [5,6].
Regarding radiation therapy, dose escalated EBRT has been found to improve clinical outcomes in multiple randomized trials [7,8,9,10,11]. However, increasing doses of EBRT may be associated with increased toxicity [12]. While biochemical and toxicity outcomes appear similar with extreme hypofractionation via stereotactic body radiation therapy (SBRT) compared to conventional techniques, long-term follow-up is lacking [13,14,15]. Given the rapid dose fall off associated with brachytherapy, one can achieve an increased BED while limiting normal tissue toxicity. Both high-dose-rate (HDR) and low-dose-rate (LDR) techniques exist, each with a unique set of advantages and disadvantages [16,17,18]. In the definitive management of prostate cancer, brachytherapy can be used as a monotherapy or as a boost with EBRT depending on the aggressiveness of the disease, with favorable outcomes compared to EBRT alone [19,20]. With equivalent oncologic outcomes between HDR and LDR techniques, quality of life differences between the two become paramount. It is also important for patients to understand what increases in toxicity they are accepting, when brachytherapy is added as a boost after EBRT [21,22]. We report our institutional QOL data for LDR monotherapy (LDR mono), HDR monotherapy (HDR mono), and EBRT with an HDR brachytherapy boost (HDR boost).
Material and methods
After institutional review board approval, the charts of 202 patients with biopsy proven and clinically localized adenocarcinoma of the prostate, treated with HDR or LDR brachytherapy between June 2012 and December 2015, were reviewed. Patients were treated either with HDR mono, HDR boost, or LDR mono radiotherapy (RT). Patient-reported outcomes (PRO) were collected at baseline and at regular intervals after treatment using the American Urological Association symptom score (AUASS) and the Expanded Prostate Index for Prostate Cancer – Clinical Practice (EPIC-CP) [23,24]. The American Urological Association developed the AUASS to determine how bothersome men’s urinary symptoms are. Each urinary symptom is given a score from 0-5. A score of 0 means the symptoms do not occur at all, while a score of 3 means men experience the symptoms about half the time. A score of 5 means the symptoms occur almost always. The AUASS consists of 7 urinary specific questions and is scored out of a total of 35 points. The EPIC-CP is divided into subdomains, including urinary incontinence (incon), urinary irritability and obstructive symptoms (irr/obs), bowel function, sexual function, and vitality. Each subdomain consists of 3 questions and is scored out of a total of 12 points, while the overall EPIC-CP quality of life score is out of a total of 60 points. Patients without baseline surveys and at least one follow-up survey, and patients who were previously treated for prostate cancer were excluded, leaving 165 patients for review.
Brachytherapy treatment
For both HDR and LDR brachytherapy, biplanar ultrasonography was performed in transverse and sagittal dimensions to identify the prostate, seminal vesicles, bladder, and rectum (Hitachi Hi VISION Avius, Tokyo, Japan). For HDR, a transperineal interstitial brachytherapy template was used under ultrasound guidance to place 5 or 6-French HDR catheters into the prostate at the appropriate depth circumferentially around the peripheral capsule of the prostate, avoiding the urethra. Computed tomography (CT) simulation was then performed to verify brachytherapy catheter depth and for treatment planning. The treatment plan was generated with comprehensive treatment planning software (Oncentra Brachy, Stockholm, Sweden). Treatment was delivered to the target volume using a remote after loaded Ir-192 source. When delivered as monotherapy, HDR dose was 13.5 Gy in 2 fractions (n = 73) or 12 Gy in 2 fractions (n = 2). When delivered as a boost after EBRT, the HDR dose was 9.5 Gy × 2 fractions (n = 38), 10 Gy × 2 fractions (n = 10), 10.5 × 2 fractions (n = 3), or 15 Gy × 1 fraction (n = 4). The doses used represent the evolution of our practice to a higher dose per fraction, with the 2-fraction regimen for both monotherapy and boost. For boost patients, a single fraction regimen was ultimately used for patient convenience [25,26,27,28,29]. For LDR, a template grid was also applied, and using the previously configured plan, palladium-103 seeds were placed under ultrasound guidance using pre-loaded needles. Following review of the real-time ultrasound dosimetry, a decision was made whether or not to supplement the preplanned seed configuration. For all LDR monotherapy patients, the prescribed dose was 125 Gy.
EBRT treatment
All patients in the HDR boost group received EBRT prior to brachytherapy. The clinical target volume (CTV) standardly consisted of the prostate and seminal vesicles, and a 0.3-1 cm margin was added to the CTV to create the planning target volume (PTV). Treatment doses included 45 Gy in 25 fractions (n = 38), 37.5 Gy in 15 fractions (n = 14), and other (n = 3). All patients were treated with either intensity-modulated radiation therapy (IMRT) or volumetric-modulated arc radiotherapy (VMAT) under daily image guidance. Treatment details for brachytherapy and EBRT are listed in Table 1.
Table 1
Patient and treatment characteristics
Statistical analysis
Three groups of prostate cancer patients treated with 3 regimens (HDR mono, HDR boost, and LDR mono) were included in the analysis. Outcomes include AUA score and EPIC-CP subdomain scores – incon, irr/obs, bowel, sexual function, vitality, and QOL. All outcome variables were treated as the continuous variables. For descriptive statistics of each outcome, time points were set-up as baseline, ≤ 2 months, 2-≤ 6 months, 6-≤ 12 months, 12-≤ 18 months, 18-≤ 24 months, 24-≤ 30 months, and > 30 months based on standard post-treatment follow-up visit times in our department. The mean and standard deviation of the outcomes of the 3 different treatment groups at different time points were calculated. Linear mixed models were performed to test whether there were any significant changes over time for each outcome and to detect whether there were any significant differences of each outcome among the different treatment groups. The significance level was set at 0.05. SAS 9.4 was used for data analyses and management.
Results
Patient characteristics
A total of 165 men with localized prostate cancer were included in the study. Thirty five patients underwent treatment with LDR mono, 75 with HDR mono, and 55 with an HDR boost. Median follow-up for all groups was 19.5 months (range, 1.1-51.97 months). The median age at treatment was 66 years (range, 48-87 years). A majority of patients had cT1c disease (n = 132), and the most prevalent Gleason score was 3 + 4 (n = 86). 85.5% of patients had a pre-treatment PSA of < 10. Patient characteristics are presented in Table 1.
Urinary function
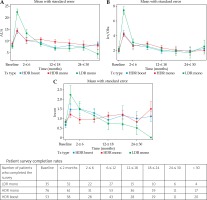
AUA score was measured at the listed time points and increased for all groups after intervention. Peak scores for all groups occurred at ≤ 2 months before improving (Figure 1). AUA score was significantly associated with time from treatment (longitudinal effect, p < 0.001). AUA score was significantly higher for the LDR group at ≤ 2 months (p < 0.0001) compared to the HDR monotherapy and HDR boost groups. Scores were not significantly different between the 3 groups at other time points. For each group, the AUA score at baseline was not significantly different from the AUA score at the > 30-month time point (all p ≥ 0.3). Irr/obs urinary symptoms were also significantly associated with time from intervention for all groups (longitudinal effect, p < 0.0001), with a peak incidence at ≤ 2 months for all groups before improving (Figure 1). Irr/obs symptoms were significantly increased for the LDR group at the ≤ 2-month time point (p < 0.0001) compared to the other 2 groups but were otherwise not significantly different between the 3 groups. For all groups, irr/obs scores were not significantly different from baseline at the > 30-month time point (all p ≥ 0.45). Incon scores peaked in the LDR group at ≤ 2 months, in the HDR mono group at > 30 months, and in the HDR boost group at 2 to ≤ 6 months (Figure 1). Rates of incon were significantly worse in the LDR group at ≤ 2 months compared to the other groups (p = 0.043). Incon was not significantly associated with time from intervention (longitudinal effect, p = 0.242). Compared to baseline, incon scores at > 30 months were not significantly different in the HDR boost and LDR groups. While the difference did reach significance in the HDR mono group (p = 0.044), the absolute difference was small, with average scores of 0.84 ± 1.12 and 1.50 ± 1.62 at baseline and > 30 months, respectively.
Bowel function
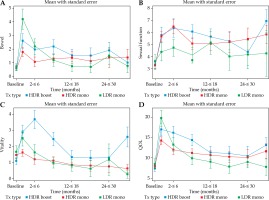
Bowel toxicity peaked for all groups at ≤ 2 months from intervention before improving and was significantly worse in the LDR group compared to the HDR monotherapy and boost groups at the same time point (p = 0.005) (Figure 2). Bowel toxicity was not significantly associated with time from intervention (longitudinal effect, p = 0.629). In addition, EPIC-CP bowel toxicity score was not significantly different from baseline at > 30 months for any group (all p ≥ 0.09).
Sexual function
Rates of sexual dysfunction were not significantly associated with time from intervention (longitudinal effect, p = 0.160) and did not significantly differ between the 3 groups at any time point (Figure 2). Compared to baseline, sexual function scores were significantly worse at the > 30-month time point in the HDR boost and HDR mono groups (both p ≤ 0.001). While numerically worse in the LDR group, this did not reach significance (p = 0.7).
Vitality/quality of life
Vitality scores were significantly worse in the HDR boost group at ≤ 2, 2 to ≤ 6, 6 to ≤ 12, and > 30 months (p = 0.024, p = 0.0003, p = 0.004, and p = 0.010, respectively) compared to the HDR mono and LDR mono groups. Vitality scores did significantly change with time (p = 0.0006) (Figure 2). In the HDR boost group, the vitality score at > 30 months was significantly worse than at baseline (p = 0.007). Analysis of QOL showed peaked scores for all groups at ≤ 2 months (Figure 2). QOL was significantly worse for the LDR group at this time point (p = 0.021). Scores were not significantly associated with time from treatment. Overall QOL score was significantly worse at the > 30-month time point compared to baseline in the 2 HDR groups (for both, p < 0.03), but did not reach significance in the LDR group (p = 0.85).
Discussion
In our study, all major functional quality of life domains were affected after treatment with brachytherapy for localized prostate cancer, including urinary, bowel, and sexual function, along with vitality. A majority of domains improved over time, with the exception of sexual function scores for all groups and urinary incontinence scores for the HDR mono group. Patients treated with LDR did have higher AUA, irr/obs, incontinence, bowel, and QOL scores acutely compared to the HDR and HDR + boost groups. Vitality scores were significantly worse in the HDR boost group both acutely and at the > 30-month time point. However, the HDR boost group did have more patients who received androgen deprivation therapy (ADT), which likely contributed to this result (Table 1).
There have been 2 recent studies comparing HDR and LDR brachytherapy. In a single-institution retrospective study, Grills et al. compared toxicity between HDR and LDR monotherapy [16]. HDR was delivered in 4 fractions, 2 times a day over 2 days. The LDR dose was 120 Gy. The HDR group experienced decreased rates of acute grade 1 to 3 dysuria, urinary frequency/urgency, and rectal pain, which is consistent with our data. In addition, the HDR group had lower rates of erectile dysfunction at 3 years after treatment. A surveillance, epidemiology, and end results program (SEER) analysis comparing LDR monotherapy, HDR monotherapy, EBRT + LDR boost, and EBRT + HDR boost was reported by Tward et al. [17]. Cumulative urinary adverse events were the highest in the EBRT + HDR boost group and lowest in the LDR group at 8 years. The risk of development of adverse treatment-related urinary events was highest in the 2 years after treatment for the LDR, EBRT + LDR, and EBRT + HDR groups before declining. The risk was greatest in the first 4 years for the HDR monotherapy group before declining. This can perhaps be applied to our finding of peak incontinence score in the HDR mono group at > 30 months.
Previous single institution series have shown that grade 3 and 4 toxicity is rare with both HDR and LDR brachytherapy [30,31,32,33,34,35]. In addition, Sanda et al. prospectively measured patient-reported QOL outcomes among patients undergoing radical prostatectomy, EBRT, or brachytherapy for treatment of localized prostate cancer [5]. In this study, patients in the brachytherapy group were exclusively treated with LDR isotopes. Those in the brachytherapy group had the lowest rates of erectile dysfunction but the highest rates of urinary obstruction/irritation. Both radiotherapy groups experienced acute bowel toxicity. As in our study, both urinary and rectal symptoms did improve with time from intervention. In fact, there was no significant difference in irr/obs symptoms at > 30 months compared to baseline. In a population-based prospective cohort study of 1,141 men, Chen et al. compared patient-reported QOL between men treated with radical prostatectomy, EBRT, and brachytherapy, and those who were elected for active surveillance [6]. In the brachytherapy group, LDR and HDR were not differentiated. Patients in the brachytherapy group had increased rates of sexual dysfunction compared to those on active surveillance at 3 and 12 months, but not at 24 months. The same trend was seen with irritation/obstructive urinary symptoms. Those in the brachytherapy group did not have higher rectal toxicity compared to those who were elected for active surveillance.
Chen et al.’s thoughtful comparative data of QOL changes with treatment also importantly reports QOL changes relative to baseline for each modality. This allows us to give context to the magnitude of change experienced after treatment with each modality. It is important to note that changes in sexual and urinary QOL were shown to deteriorate in the active surveillance cohort over the course of the study. Finally, the changes in Chen et al.’s brachytherapy cohort were also seen in our study in similar magnitude. We feel it is important to put into context and acknowledge that there are more QOL changes associated with multimodality therapy (HDR boost in our study), and that these are necessary for appropriate risk group adjusted treatment. HDR as a boost and ADT are reserved for our highest risk, yet still localized patients. Our data revealed multimodality therapy may have more treatment-related side effects (notably significantly worse vitality scores), yet we note this is consistent with other studies of multimodality care in the treatment of prostate cancer. For instance, the use of definitive radiotherapy with dose escalation, treatment of elective lymph nodes, and with ADT, and the addition of EBRT after prostatectomy are beneficial, well proven interventions [5,6,36]. Thus, we accept the relative causative QOL changes, if present, when treating with HDR boost as well. In addition, absolute changes in QOL scores were small in our study.
Limitations to our study include its retrospective nature and limited follow-up. Furthermore, patients were treated by a single brachytherapist, which may limit application to the general population. Still, compared to the above studies, ours is the first comprehensive QOL analysis comparing HDR monotherapy, LDR monotherapy, and EBRT + HDR boost in the modern era. Also, follow-up in other patient-reported QOL studies is similar to our own. For instance, Sanda et al. report 24-month follow-up in 730 of the > 1,200 patient enrolled [5]. Thus, our study continues to be unique in its report of patient-reported QOL outcomes. As it is unlikely a randomized trial will be conducted comparing LDR and HDR brachytherapy for localized prostate cancer, retrospective reviews are used to inform decision making. LDR is the most widely employed technique in the United States given the long follow-up data, acceptable toxicity rates, and convenient delivery in one procedure. However, HDR brachytherapy better lends itself to dose escalation given the flexibility in treatment planning afforded by CT-based post-implant dosimetry. In addition, there are now multiple studies with long-term follow-up published, giving HDR a place as one of the primary treatment modalities for localized prostate cancer [31,32]. More convenient fractionation schedules for HDR have also been studied and are widely used in practice [25,30,37]. In our study, patients receiving HDR brachytherapy had lower acute urinary and rectal toxicity compared to the patients receiving LDR, even when combined with EBRT. However, both techniques have been shown to lead to excellent biochemical control rates, and long-term toxicity was similar in our study.
In conclusion, given the low rates of toxicity associated with LDR and HDR brachytherapy, which largely improve with time from intervention, these techniques remain appropriate alternatives to prostatectomy for patients with localized prostate cancer.