Purpose
Cervical cancer remains the second leading cause of death in women between the ages of 20-39 years [1]. The incidence of cervical intra-epithelial neoplasia and cervical cancer has declined since the advent of effective screening and human papillomavirus (HPV) vaccination [2-5]. Despite this decline, there would be 13,800 new diagnoses of cervical cancer in the United States during the year 2020 [1]. The standard of care for definitive management of locally advanced and node-positive cervical cancer remains external beam radiation therapy (EBRT) with concurrent chemotherapy, followed by a brachytherapy boost.
Brachytherapy delivers high radiation doses to a tumor while minimizing dose to adjacent organs at risk (OARs), including the bladder, rectum, and small bowel. Inclusion of brachytherapy into a cervical cancer patient’s treatment plan is associated with improved progression-free survival (PFS) and overall survival (OS) [6-8]. Recent attempts to substitute stereotactic ablative radiation therapy (SAbR) as an alternative for intracavitary or interstitial brachytherapy boost, demonstrated poor outcomes in study population [9]. Despite this evidence, the use of brachytherapy has declined in the United States, with concurrent increases in use of EBRT or SAbR boost [6, 10]. There are many potential causes for underutilization of brachytherapy, including unfavorable reimbursement policies, use of EBRT boost, patients’ access issues, insufficient resident training, and intensive workflow [6, 11-15].
After appreciating the decline in brachytherapy nationally, our group explored the statewide use of brachytherapy in California. We found that San Diego and Imperial Counties, which serve over 3.5 million people [16], account for 8.6% of California’s cervical cancer cases according to the California Cancer Registry (CCR). However, only 44% of patients in San Diego and Imperial County with locally advanced cervical cancer receive brachytherapy, compared to 55.8% nationally [17]. Given the limitations of larger datasets, such as SEER, NCDB, and the California Cancer Registry to obtain detailed information on the potential rationale for low utilization trends, we sought to explore our local barriers to care and referral patterns. The purpose of this study was to investigate brachytherapy practice and referral patterns from radiation oncologists in the Western United States border region.
Material and methods
A 28-item survey was electronically mailed in May 2020 to 28 radiation oncologists in San Diego and Imperial Counties, who actively treat patients with gynecologic malignancies. Four additional e-mail reminders were sent between June through July 2020. The survey contained questions regarding the provider’s practice, brachytherapy training, practice and referral patterns, factors affecting preference between EBRT and brachytherapy, barriers to brachytherapy delivery, changes to brachytherapy practice during the COVID-19 pandemic, and 3 case scenarios (Appendix 1). The case scenarios were identical to those previously sent to the American Brachytherapy Society (ABS) listserv of active members and published by our group, to allow for comparison of responses [13].
Provider responses were compiled, and descriptive statistics were used to assess relative frequency distributions. This study was reviewed and approved by the University of San Diego Institutional Review Board (HRPP, No. 200156).
Results
Survey population
Seventeen (61%) of the 28 physicians completed the survey, of which 11 (65%) were identified as males and 6 (35%) were identified as females. Participating physicians had an average of 15 years of experience as an attending physician (range, 1-32 years). Additional characteristics including details of practice location, affiliation, and patients’ population are presented in Table 1.
Table 1
Practice characteristics of participating physicians
Brachytherapy training
All physicians (100%) reported they received training in cervical cancer brachytherapy during residency. One physician reported additional cervical cancer brachytherapy training during fellowship. The length of brachytherapy training ranged from 2 to 36 months, with a median of 6 months. One physician reported completing less than 10 brachytherapy cases prior to independent practice, 8 (47%) reported 10-20 cases, 4 (24%) reported 21-50 cases, and 4 (24%) reported more than 50 cases. Seven (41%) respondents strongly agreed, and eight (47%) agreed with the statement “I received adequate cervical cancer brachytherapy training”. One physician felt neutral, and another disagreed with the statement. When asked to evaluate the statement “I feel comfortable performing cervical cancer brachytherapy”, eight physicians (47%) strongly agreed, 4 (24%) agreed, 3 (18%) were neutral, and 2 (12%) strongly disagreed.
After becoming an attending physician, five (29%) of the physicians completed additional training courses in brachytherapy. The majority of doctors (77%) were not interested in additional brachytherapy training. Of the three (23%) physicians who were interested in additional training, topics of interest included contouring and treatment planning (n = 2), intracavitary procedures (n = 1), and interstitial or hybrid procedures (n = 3).
Brachytherapy practice patterns
Of the 17 respondents who deal with gynecologic malignancies, 13 (76%) treat cervical cancer. When asked to report the number of intact cervical cancer brachytherapy cases (patients) performed per year, 33% of the physicians reported 1-5 cases, 33% reported 11-20 cases, 11% reported 11-20 cases, and 22% reported more than 20 cases. The number of intact cervical cancer brachytherapy cases (patients) performed to date was reported to be 11-50 for 11% of the physicians, 51-100 for 33% of the physicians, and over 100 for 56% of the physicians.
For those physicians who perform brachytherapy, difficult cases were discussed with local colleagues (22%), group partners (78%), or national colleagues with more expertise (33%). If faced with a difficult cervical cancer brachytherapy case that could lead to suboptimal outcomes or dose distribution under their care, 75% would refer outside their practice, 13% would treat with SAbR boost, and 13% would proceed as usual.
When asked to identify scenarios when EBRT or SAbR boost would be recommended instead of brachytherapy for locally advanced cervical cancer, the physicians reported considering advanced age, multiple comorbidities, palliative intent, inability to place an intrauterine tandem, patients’ preference, or inability to seek care at a referral facility. Two physicians reported they would never perform EBRT or SAbR boost over brachytherapy.
Changes to practice in the era of COVID
The COVID-19 pandemic led to significant changes in many procedural and surgical patients’ volumes. When asked about changes to EBRT practices due to the pandemic, most (78%) reported no change. Changes in practice included delayed start of therapy and increased hypofractionation when possible. No changes were reported for brachytherapy practices other than the addition of pre-procedural testing for coronavirus.
Brachytherapy referral patterns
Two (15%) physicians reported personally treating all cervical cancer patients with brachytherapy. The others referred some (54%) or all (31%) patients for brachytherapy, with majority of referrals for patients considered to require hybrid or interstitial brachytherapy implants (78%) at the time of starting EBRT. Additional reasons for referrals included concern with sedation safety. Referrals were also influenced by insurance status (25%) and patients’ resources or logistics (13%). Physicians who did not personally perform brachytherapy on every cervical cancer patient cited low number of annual cases (n = 4), inadequate training (n = 1), inadequate maintenance of skills (n = 3), lack of equipment (n = 5), preference for EBRT (n = 1), time to perform brachytherapy (n = 2), and shared group coverage of cervical cancer patients (n = 1).
Perceived barriers to increasing brachytherapy utilization
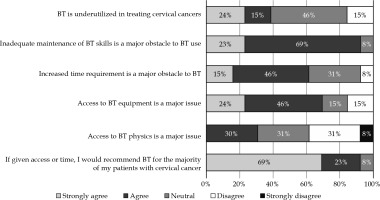
A series of six statements were presented to the respondents to assess their perception of brachytherapy utilization and potential barriers (Figure 1). Approximately one-third of the physicians strongly agreed or agreed with the statement “Brachytherapy is underutilized in treating cervical cancers”, while 46% were neutral. Almost all (92%) strongly agreed or agreed that “Inadequate maintenance of brachytherapy skills is a major obstacle to brachytherapy use”. Many (61%) strongly agreed or agreed that “Increased time requirement is a major obstacle to brachytherapy”. Most (69%) strongly agreed or agreed with the statement “Access to brachytherapy equipment is a major issue”, and only 31% agreed that “Access to brachytherapy physics is a major issue”. Almost all physicians (92%) strongly agreed or agreed with the statement “If given access or time, I would recommend brachytherapy as one of the treatment modalities for the majority of my patients with cervical cancer”.
Case scenarios
The survey then presented three case scenarios to the physicians and asked them to choose their top treatment option.
Case 1: “A 40-year-old female with 6 cm FIGO stage IIB squamous cell cervical cancer, which invaded into left lateral parametrium without lymph node disease. After chemoradiation therapy to the pelvis to 45 Gy, what type of boost would you use after EBRT to the whole pelvis?”.
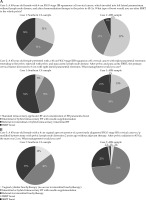
The survey responders were approximately evenly split between standard intracavitary applicator brachytherapy and consideration of external beam parametria boost (31%), interstitial or hybrid intracavitary brachytherapy with needle supplementation (31%), and referral for interstitial or hybrid intracavitary brachytherapy (38%) (Figure 2A). No physicians chose intensity modulated radiation therapy (IMRT) or SAbR boost. In comparison, the ABS responses indicated that 55.6% preferred standard intracavitary applicator brachytherapy and consideration of external beam parametria boost, 37.0% chose interstitial or hybrid intracavitary brachytherapy with needle supplementation, and only 6.2% selected referral for interstitial or hybrid intracavitary brachytherapy.
Fig. 2
A) Comparison of responses to case scenarios 1 and 2 by the American Brachytherapy Society (ABS) respondents (n = 81) and Southern California respondents (n = 13). B) Comparison of responses to case scenario 3 by the ABS respondents (n = 81) and Southern California respondents (n = 13)
Case 2: “A 45-year-old female presented with a 10 cm FIGO stage IIIB squamous cell cervical cancer with right parametrial extension extending to the pelvic sidewall with pelvic and para-aortic lymph node disease. After pelvic and para-aortic EBRT, the primary cervical tumor decreased to 6 cm with right lateral parametrial extension. What management would you use?”.
Approximately half (54%) of the physicians chose referral for interstitial or hybrid intracavitary brachytherapy, while another 38% chose interstitial or hybrid intracavitary brachytherapy with needle supplementation. Only 8% would proceed with standard intracavitary applicator brachytherapy and consideration of external beam parametria boost, and no responders chose IMRT or SAbR boost (Figure 2A). These preferences were similar to the ABS responses, which showed that 69.1% chose interstitial or hybrid intracavitary brachytherapy with needle supplementation as their preferred mode of management.
Case 3: “A 45-year-old female with a 4 cm vaginal apex recurrence of a previously diagnosed FIGO stage IB1 cervical cancer s/p modified hysterectomy with pelvic lymph node dissection 2 years ago without adjuvant therapy. After pelvic radiation to 45 Gy, the mass was 2 cm. What management would you apply?”.
Most responders chose interstitial or hybrid intracavitary brachytherapy with needle supplementation (46%) or referral for interstitial or hybrid intracavitary brachytherapy (38%), while 15% decided on vaginal cylinder brachytherapy (Figure 2B). No physicians chose to proceed with IMRT or SAbR boost. Compared to the ABS physicians, interstitial or hybrid intracavitary brachytherapy with needle supplementation was chosen by 58.0% of the participants.
Discussion
This study investigated regional cervical cancer brachytherapy practice patterns of academic and community radiation oncologists in a Western United States border region. By examining local practice patterns, we sought to identify barriers to referral that otherwise are not able to be collected from large national databases. We found that most providers recognize the importance of brachytherapy in the care of locally advanced cervical cancer, and would recommend it for a theoretical patient. However, only one-third of providers thought that brachytherapy was underutilized in clinical practice. Most of the physicians received adequate training during residency, with all but one reporting more than ten cases, but several were not comfortable performing brachytherapy in practice. Almost all reported that inadequate maintenance of skills was the major barrier to brachytherapy use. While some attended additional training courses in brachytherapy following residency, the majority were not interested in additional brachytherapy training. The COVID-19 pandemic contributed to delays in initiating EBRT and increased pre-procedural testing prior to brachytherapy, but overall did not cause changes in brachytherapy practice.
The evidence for use of brachytherapy in the treatment of cervical cancer extend over decades, and numerous studies have demonstrated that brachytherapy is strongly correlated with improved local control and survival [6, 8, 18-21]. The American Brachytherapy Society (ABS) and Society for Gynecologic Oncology (SGO) continue to advocate for brachytherapy as an essential component of definitive treatment for locally advanced cervical cancer [11], and the National Comprehensive Cancer Network (NCCN) explicitly states that “SBRT is not considered an appropriate routine alternative to brachytherapy”. In addition, the Commission on Cancer has developed quality reports that measure the use of brachytherapy in patients treated with primary radiation with curative intent in cervical cancer, and radiation therapy package time of 60 days from initiation to completion [22]. However, underutilization of brachytherapy persists in the United States as a whole [17] as well as within our Southern California border region.
Our findings regarding brachytherapy training during residency reflect those of the Association of Residents in Radiation Oncology (ARRO) and ABS [13, 23-25] in that all physicians received training, but some (30%) felt that the training was not adequate for independent practice. This feedback appears to indicate recent Accreditation Council for Graduate Medical Education (ACGME) increase in the minimum required brachytherapy procedures to at least seven interstitial and fifteen intracavitary procedures, of which no more than five can be cylinder insertions [26]. While these requirements may ensure that residents have broader exposure to brachytherapy during training, some may argue that 22 procedures over a four-year program remains low for a physician expected to perform independently upon graduation, when compared to the minimum caseloads mandated for most interventional and surgical training programs. Data from recent residents indicate an association between number of brachytherapy cases performed and confidence in starting a brachytherapy practice [12]. Externships during residency and fellowships after residency can provide further hands-on opportunities to gain expertise, but may be impeded by time constraints, lack of standardization, or financial disincentives.
We also found that if procedural skills were not maintained post-graduation, most physicians (77%) were not interested in additional brachytherapy training. The physicians participated in our survey had an average of 15 years of experience in independent practice, but the low enthusiasm for further training after a lapse in practice, echoes that of recent senior residents [12]. While it is expected for some physicians to gravitate towards specific disease sites or procedural vs. non-procedural practice, these findings highlight the importance of early support and mentorship for those who plan to treat patients with brachytherapy after residency. The ABS has committed to training the next generation of brachytherapists via the 300 in 10 initiative, with the goal of 30 competent brachytherapists per year over the upcoming 10 years. Areas of focus include an online brachytherapy curriculum, simulation training workshops, short-term focused fellowships at the ABS certified centers, and competency evaluations. There is also a longitudinal mentorship program for early-career physicians, #NextGenBrachy. These programs to facilitate the transition between residency and independent practice may mitigate some loss of interest from an initial lapse in practice after graduation.
Our survey showed additional barriers to brachytherapy use, independent of inadequate training or maintenance of skills. These include patients’ preference or difficulties with the referral process, which may hinge on insurance status and patients’ resources or logistics. Social and financial barriers to appropriate treatment only enhance the effects of existing disparities in cervical cancer screening, where there are disproportionately low rates of Pap smear screening for Black and non-English-speaking immigrant women [17, 21, 27, 28]. In addition, the current payment system disincentivizes highly cost-effective treatments, such as brachytherapy, although a suitable alternative treatment has yet to be found. A major barrier to brachytherapy utilization is the additional time requirement for procedures, with 61% of the physicians indicating it as a major obstacle. Current reimbursements do not adequately reflect this effort, as attending time per relative value unit (RVU) for brachytherapy is approximately four-fold higher than for EBRT [29]. As the field transitions to value-based care via the upcoming radiation oncology alternative payment model (RO-APM) [30], it is imperative that referral for brachytherapy by a physician who does not provide the technique is encouraged rather than penalized as a “duplicate service”. The unintended consequences of this reimbursement strategy include decreased resident exposure, which only compounds the issue of inadequate training.
There were several limitations of this study, including its’ survey-based nature, which was susceptible to self-report bias. The sample size reflected the community of practicing radiation oncologists in the Southern California border counties; therefore, our results may not be generalizable to other regions of the state or country. While the overall rate of brachytherapy usage in the region was low at 44%, all the participants reported that they either performed brachytherapy or referred for brachytherapy. However, the response rate of 61% was slightly higher than the typical response rate of healthcare professionals [31], and survey respondents were diverse in prior training, practice environments, and years in practice. As recent surveys determined the residents’ perspectives, we limited study participants to attending radiation oncologists. Additionally, a qualitative semi-structured interview could have been used to capture in-depth responses for key findings.
Conclusions
The results of this survey underscore the barriers to brachytherapy implementation for definitive treatment of cervical cancer in a regional area of the United States. There are currently no acceptable alternatives to brachytherapy for delivering a safe and effective radiotherapy boost in patients undergoing curative treatment for locally advanced cervical cancer, yet over 10% of the physicians in our sample would perform SAbR boost when faced with a difficult brachytherapy case. While the ABS 300 in 10 initiative provides additional support for early-career brachytherapists via training fellowships, supplementary approaches should focus on augmenting patients’ resources to mitigate logistical challenges and streamlining referral processes to local experts to facilitate timely treatment completion. In addition, systemic revision of existing reimbursement policy should appropriately reflect brachytherapy’s impact on survival in the definitive treatment for patients with cervical cancer.