Purpose
Medulloepithelioma is the second most common type of pediatric intra-ocular tumors. It commonly arises from ciliary body, and it is generally diagnosed in the first decade of life [1]. Management options for medulloepithelioma include enucleation, resection, or radiotherapy. Eye salvage therapy with brachytherapy plaque could be an effective option in cases of small to medium size tumors [2]. Herein, we report a case of medulloepithelioma treated with brachytherapy plaque after fine needle aspiration biopsy (FNAB) during the same interventional procedure.
Case report
A 12-month-old girl was referred to our Ophthalmology Department due to iris mass in left eye (OS). Family and medical history were negative, and parents did not report any subjective complaint of the child. The left eye anterior segment examination revealed a pinkish-white iris mass at 2 o’clock to 4 o’clock meridians OS, with no satellites lesions (Figure 1A). Intra-ocular pressure determined by I-Care rebound tonometer was 12 mmHg, and fundoscopy did not exhibit any significant abnormalities. Examination of the right eye and systemic physical examination were typical. A preliminary diagnosis of iris xanthogranuloma was established.
Fig. 1
A) At presentation, anterior segment examination of the left eye with pinkish iris mass. B) Color fundus photography demonstrating peripheral retinal lesion at 24-month-old. C) Ultrasound B-scan showing a mass lesion with moderate to high internal reflectivity, arising from the ciliary body region of 6.0 × 5.0 mm (asterisks indicate apical height measurement), without retinal detachment, corresponding to the peripheral retinal lesion in the previous image. D, E) Enlargement of ciliary body tumor to 7.0 mm of apical height (asterisks), and intra-tumoral cysts (yellow arrow) showed by ultrasound B-scans in the 26-month-old patient, 14 months after initial presentation
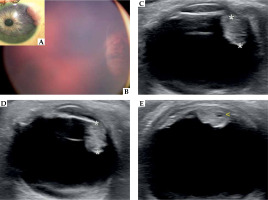
The patient underwent follow-up visits every 4 months for one year. Then, in a periodic examination under anesthesia, a retinal whitish peripheral lesion beneath the iris mass was observed (Figure 1B). B-scan ultrasonography imaging identified a ciliary body mass, of 6.0 mm × 5.0 mm (Figure 1C). A biopsy by anterior chamber paracentesis was obtained, and revealed fibrous tissue with negative staining for S-100, CD1a, and langerin. Management with close observation was indicated.
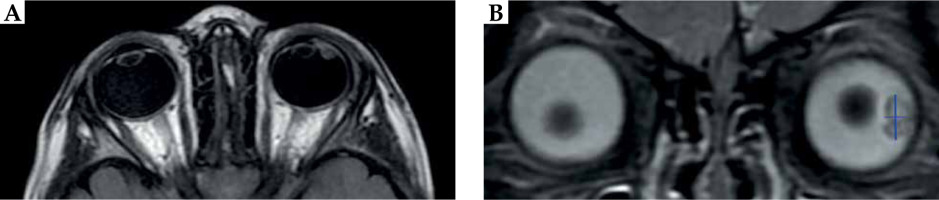
Two months later, at 26-month-old, the parents indicated that the girl had been suffering from recurrent acute episodes of left hyperemia and intense pain, which had increased in frequency and intensity during previous days. The retinal images showed a new relevant retinal lesion enlargement, confirmed by B-scan ocular ultrasonography with 15 Mhz (Acuson 18L6HD linear ultrasound probe, Siemens), which also revealed intra-tumoral cysts (Figures 1D, E). Orbital magnetic resonance imaging (MRI) demonstrated similar observations, exhibiting an heterogenous lesion, which was hyperintense to vitreous on T1-weighted sequence and hypointense on T2-weighted STIR sequence, arising from the lateral ciliary body, with posterior axis lens displacement. Measurements obtained were 7.0 mm (craniocaudal direction) × 5.0 mm (thickness from the outer sclera) in the coronal plane, and 4.4 mm (antero-posterior oblique direction) in the axial plane (Figure 2A, B). All these findings were highly suggestive of ciliary body medulloepithelioma. A comprehensive extension study, including MRI, lumbar puncture, and DICER-1 gene analysis did not reveal any additional abnormality.
Fig. 2
MRI images on T2 weighted STIR sequences of the patient with (A) axial and (B) coronal sections, showing an irregular soft tissue lesion within the left globe occupying the vitreous region. The lesion was of mixed signal intensity, measuring 7.0 mm in the cranio-caudal direction and 5.0 mm thickness (blue lines). No obvious macroscopic retrobulbar extension was visualized
In order to assess the origin of the lesion, FNAB of ciliary body mass was performed with trans-scleral approach under general anesthesia. A 3-mm square scleral flap to a depth of approximately 80% was dissected, and a short 27 gauge needle attached to short tubing was inserted through the scleral bed. Extemporaneous anatomo-pathological study demonstrated the presence of small round blue cells, compatible with malignant neoplasm, including medulloepithelioma.
Brachytherapy procedure
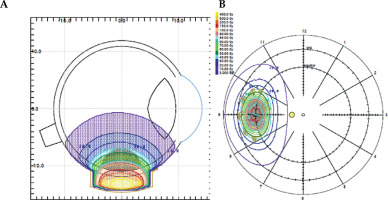
Treatment with a iodine-125 brachytherapy plaque was undertaken in the same interventional procedure. Trans-scleral illumination was applied to assess tumor margins, and a safety margin of 2 mm was used, after taken into consideration characteristics of the plaque and tumor measurements [3]. The lesion was treated using a plaque brachytherapy with total radioactivity of 13.5 mCi, which was distributed in 5 seeds. Treatment planning system used was Plaque Simulator (v. 5.3.6) (Astrahan 1990) [4], with all correction factors included. Gold plaque used was COMS10 [5], with 11 mm of nominal diameter, loaded with 5 iodine seeds. Iodine-125 (125I) seeds loaded on COMS plaque were model IsoSeed® I25.S16 from Eckert & Ziegler (Berlin, Germany). Source reference air kerma rate at the time of implantation was 3.429 µGyh-1m2 (equivalent to an apparent activity of 2.70 mCi). Prescription point was at tumor apex, 5 mm in total (4 mm plus 1 mm of the sclera). Fundus diagram and dose distribution are presented in Figure 3. The patient was hospitalized during the course of brachytherapy, and the plaque was removed after 124.4 hours. A prescription dose of 40 Gy at tumor apex was prescribed [6], and was delivered to the tumor apex at a dose rate of 0.32 Gy/hour. The external sclera received a maximum of 282 Gy, with a dose-rate of 2.27 Gy/hour.
Post-operatively, the tumor showed regression over a period of 10 months, with complete disappearance of ocular pain episodes. MRI demonstrated a decrease in the tumor measurements: 6.0 mm (cranio-caudal direction) × 4.3 mm (thickness from outer sclera) in the coronal plane, and 3.4 mm (antero-posterior oblique direction) in the axial plane. Fifteen months after brachytherapy treatment, visual acuity was 20/20 in each eye. The patient did not report any symptoms, such as blurred vision or floaters; although she does not present extensive communication abilities due to age. Examination of the anterior segment remained unremarkable. Follow-up examinations are continuing.
Discussion
Intra-ocular medulloepithelioma is a congenital, non-hereditary tumor, arising from primitive ciliary medullary epithelium, or inner layer of the optic cup, prior to its’ differentiation into adult derivatives. Patients are usually asymptomatic in early stages of disease. However, red eye, blurred vision, or pain can occur due to tumor enlargement with lens subluxation, or by rotation of the lens-iris diaphragm with angle closure [7], which could be compatible with the episodes of pain presented by our patient.
Diagnosis needs a high index of suspicion in children with a retinal whitish mass. They display clinical characteristics similar to retinoblastomas, such as leukocorias and intra-ocular endophytic masses, and are difficult to clinically differentiate on some occasions [8]. Contrary to retinoblastomas, medulloepitheliomas are most often located in the peripheral retina and do not present calcifications. Intra-tumoral cysts have been shown in 50% of cases [9], and MRI typically reveals a ciliary body mass that is iso-hyperintense to vitreous on T1 and hypointense on T2 [10, 11], which would be consistent with our radiologic findings. Moreover, as tumor can extend into the anterior chamber, it can be misdiagnosed as iris xanthogranuloma. Other entities that should be included in the differential diagnosis are persistent fetal vasculature, ocular toxocariasis, Coats disease, or Fuchs adenoma [2]. Systemic prognosis is favorable if there is no extra-ocular extension and orbital involvement. It is important to highlight that diagnosis of ciliary body medulloepithelioma is a major indication for DICER-1 genetic testing [12]. In addition to increased cancer risks for pleuropulmonary blastoma, individuals with pathogenic germline DICER-1 mutations may also develop ciliary body medulloepithelioma, lung cysts, Wills’ tumor, genitourinary rhabdomyosarcoma as well as neuroblastoma [13].
Medulloepithelioma can be treated with local excision, enucleation, and/or radiation depending on the size of the tumor [14, 15]. Regarding eye-sparing therapies, local resection is usually insufficient and the recurrence rate is high [16], whereas treatment with brachytherapy plaque has been found to contribute to tumor regression in small to medium size tumors [17, 18]. Brachytherapy is the preferred radiation treatment modality for various intra-ocular tumors, and allows organ preservation with sparing of all or partial visual acuity [19]. Indeed, Shields et al. reported a 6-case series of patients with medulloepitheliomas, undergoing brachytherapy treatment successfully, from January 1970 to December 2017. Five of the six patients were < 12 years-old, and all tumors had a basal diameter of < 14 mm and < 11 mm thickness. At a mean follow-up of 59 months, 5 eyes showed regression without recurrence [6]. Radioactive sources are placed directly onto or around the tumor with the aid of episcleral plaques. Various sources have been used over the years, with 125I being one of the most commonly reported [20, 21].
In our case, prompt initiation of treatment was mandatory after the commencement of clinical symptoms and increase of tumor size. After presentation of this case at the Tumor Board of our center, we considered the possibility of an intra-operative brachytherapy treatment, preceded by an extemporaneous anatomo-pathological study as a novel therapeutic approach, with aiming to initiate treatment rapidly and to avoid tumor seeding. The approval was obtained from a local ethics committee and informed consent was signed by the parents. Although rare, the possibility of seeding malignant cells outside the eye after tumor’s biopsy is of special concern, and some authors suggested that the risk of tumor seeding in medulloepithelioma biopsy should default to the risk-equivalent of retinoblastoma, until more information is available. Nonetheless, in some previous cases, FNAB was used to provide histologic confirmation of medulloepithelioma diagnosis [6, 18, 22-25]. Additionally, it is well-known that both resection with safety margins and radiotherapy or radiofrequency application after biopsy, may prevent the risk of malignant cell seeding in some extra-ocular tumors [26-28]. In cases of ocular tumors, it is recommended not to disturb the biopsy site in order to reduce the risk of seeding and metastases [29]. For that reasons and due to high clinical suspicion of medulloepithelioma with compatible malignant neoplasm cells presence, the brachytherapy plaque was positioned following the FNAB procedure, with meticulous supervision of the biopsy site.
In this way, intra-operative brachytherapy after FNAB in selected ocular tumors may be an adequate therapeutic option. It can also offer the advantage of a shortened interval between biopsy and radiotherapy with adequate surveillance of biopsy site to minimize cells seeding risk, and the avoidance of a second invasive procedure under general anesthesia, which is especially beneficial in infants and adolescents patients.
To the best of our knowledge, this is the first case of medulloepithelioma treated with brachytherapy plaque after an extemporaneous anatomo-pathological examination in children with favorable response. Longer follow-up and further research are necessary before intra-operative brachytherapy following FNAB to be considered the standard of care.