Purpose
Locally recurrent breast cancer (LRBC) is generally defined as recurrence involving the chest wall, axillary lymph nodes, and supraclavicular lymph nodes on the ipsilateral side of the primary lesion. Local recurrence often represents the initial manifestation of treatment failure in breast cancer, with an incidence ranging from 4% to 32%. Even after adjuvant radiotherapy (RT), the local recurrence rate remains approximately 5% to 15%, and nearly 80% of recurrences typically occur within the first 5 years following initial treatment [1], among which about 10% are deemed inoperable.
Locally recurrent breast cancer should be tailored to the size, location, and type of recurrent tumors, as well as the initial treatment received, to select an appropriate therapeutic strategy. The most frequently employed treatment modalities for LRBC include surgery, stereotactic radiotherapy, chemotherapy, and hormonal therapy [2]. However, these therapeutic modalities still have inherent limitations: certain locally recurrent breast tumors are not amenable to complete resection; systematic reviews of randomized trials have demonstrated that chemotherapy fails to yield significant therapeutic benefits; and some patients do not respond to endocrine therapy. In general, radiation therapy for localized recurrent breast cancer can achieve more durable local control. Specifically, the 5-year and 10-year survival rates after radiotherapy for patients with localized recurrence are 44% and 25%, respectively; the survival rate for isolated chest wall recurrence reaches 66%, while that for lymph node recurrence is 43%. However, due to the risk of exceeding radiation tolerance limits, external beam radiotherapy may increase the incidence of both acute and late toxicities. Thus, alternative forms of local therapy warrant consideration.
In recent years, computed tomography (CT)-guided iodine-125 (125I) seed brachytherapy has garnered increasing attention, with several studies reporting its efficacy in controlling local recurrence across various tumor types, including head and neck tumors and prostate malignancies [3-5]. These studies have demonstrated that 125I seed implantation can deliver an adequate radiation dose to the tumor while minimizing damage to adjacent normal tissues.
Therefore, this study retrospectively analyzed the clinical efficacy of 125I seed brachytherapy in 68 patients with unresectable locally recurrent breast cancer who received treatment at our institution between January 2018 and October 2023.
Material and methods
Research object
A retrospective analysis was performed on the clinical data of 90 patients with LRBC admitted to our department between January 2018 and October 2023, all of whom had unresectable locoregional recurrent breast cancer or refused surgical resection (Figure 1 and Table 1). Only patients with a minimum follow-up duration of 6 months were included in the analysis, with a final cohort of 68 female patients. The protocol of this study was reviewed and approved by the Institutional Ethics Committee of our hospital (Ethics Approval No.: YL2021-07). All patients consented to undergo 125I seed brachytherapy and provided written informed consent, which was signed by either the patients themselves or their authorized representatives.
Table 1
Clinical data of 68 patients with LRBC
Inclusion criteria:
Patients with primary breast cancer confirmed by pathological examination (either via surgical resection of the primary tumor or puncture biopsy), with locoregional recurrence confirmed by imaging or pathology.
Patients with unresectable or difficult-to-resect metastatic lesions; those who refuse surgical resection as treatment; those with metastatic lesions not previously treated with other local therapies; or those with poor general condition that may preclude tolerance of major surgical procedures.
Expected survival time of ≥ 3 months.
No severe coagulation dysfunction, and anticoagulant therapy was discontinued at least 5-7 days prior to implantation.
Exclusion criteria:
Comorbidity with other active malignancies.
Interval since last radiotherapy of less than 3 months.
Poor general condition or presence of malignant cachexia.
Incomplete clinical data or loss to follow-up.
Some of the included patients had a history of surgery, adjuvant chemotherapy, hormonal intervention, and external beam radiotherapy (EBRT). The prior treatments for locally recurrent lesions were as follows: 1. Chemotherapy: 61 patients received chemotherapy, with anthracycline- and paclitaxel-based regimens as the mainstay. 2. Radiation therapy: 61 patients underwent radiation therapy using 60Co, linear accelerator-derived high-energy X-rays, or electron beams. The irradiation fields primarily included the supraclavicular region and chest wall, with the total radiation dose amounting to 50 Gy. 3. Endocrine therapy: 39 patients received endocrine therapy, including tamoxifen, toremifene, or third-generation aromatase inhibitors. For all patients with a history of initial radiotherapy, the interval between the completion of initial radiotherapy and salvage low-dose-rate (LDR) brachytherapy was no less than 1 year.
Instruments and equipment
Iodine-125 seed implantation scanning equipment: A 64-slice CT scanner integrated with GE Discovery VCT PET/CT (GE Healthcare, USA) was used for routine scanning.
Implantation instruments: Implantation guns and pushers were procured from Xiangshan, Zhejiang Province, China; puncture needles (15-20 cm × 18G) were supplied by Hakko (Japan).
Treatment planning system (TPS): A three-dimensional radiation implantation planning system provided by Beijing Feitian Zhaoye Technology Co., Ltd. was used.
Seed source: 125I seeds were produced and supplied by Beijing Atomic High-Tech Co., Ltd.
Methods
125I seed brachytherapy
One week prior to the procedure, enhanced CT scans were imported into the TPS for designing the needle insertion paths, determining the number of seeds to be implanted, and arranging the puncture needles, among other parameters. Preoperative laboratory examinations, including blood tests, liver function assessments, coagulation profiles, and infectious disease screenings, are conducted within 72 hours before the surgery to evaluate procedural risks and ensure safety. Depending on the lesion location, patients were positioned appropriately, and an extracorporeal localization grid was placed on the skin corresponding to the lesion area. The implantation procedure was performed under CT guidance with local anesthesia. CT scanning was conducted to locate the lesion and determine the puncture points, and needles were placed layer by layer in accordance with the TPS plan. 125I seed implantation was executed with dosimetric parameters including D90, V100, and V150, which covered the planning target volume (PTV). The PTV was defined as the gross tumor volume (GTV) plus a margin of 0.5-1.0 cm. Intraoperatively, the position and orientation of the implantation needle were adjusted based on real-time CT scans to ensure that the needle passed through the center of the tumor, extending 0.5 cm beyond the tumor edge. Immediately after the procedure, a CT scan was performed to assess the spatial distribution of the seeds. If dosimetric cold spots were identified, additional seeds were implanted as needed. Intraoperatively, patients’ vital signs were monitored, and their mental status, pain levels, and respiratory function were observed. Following the procedure, pressure was applied to the puncture site for 10-20 minutes, and the patient’s condition was continuously observed. Postoperative CT scan images were imported into the TPS, and the actual tumor dose was derived from the dose-volume histogram (DVH).
Systemic treatment
Following 125I radioactive seed implantation therapy, 31 out of 68 patients received 4-6 cycles of chemotherapy, 10 patients received endocrine therapy alone, 19 patients underwent targeted therapy, and the remaining 8 patients received no antitumor agents. Chemotherapy and endocrine therapy were administered in accordance with the latest guidelines from the National Comprehensive Cancer Network (NCCN) [6].
Observation indicators and efficacy evaluation criteria
Follow-up for the 68 patients commenced immediately after 125I seed brachytherapy, with assessments scheduled at 3 days, 4 weeks, 8 weeks, and 12 weeks post-treatment. Subsequently, follow-up was conducted every 2 months for the first 2 years, and every 6 months thereafter. All patients underwent enhanced CT scans and hematological tests (including complete blood count, liver and renal function, and electrolyte levels, among others) within 3-5 weeks after the procedure. The primary endpoints were objective response rate (ORR) and local control rate (LCR), while secondary endpoints included clinical benefit, overall survival (OS), local progression-free survival (LPFS), and the incidence of adverse events. OS was defined as the time from seed implantation to the date of death or the end of follow-up. Tumor response was assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST) [7], which includes complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), defined as per standard criteria. The ORR was computed as the percentage of patients achieving CR or PR relative to the total number of cases [(CR + PR)/total cases × 100%], while the clinical benefit rate (LCR) was calculated as the percentage of patients with CR, PR, or SD among all cases [(CR + PR + SD)/total cases × 100%].
Intraoperative and postoperative complications were recorded in accordance with the Radiation Therapy Oncology Group (RTOG) acute radiation toxicity grading criteria. Patients were monitored for adverse reactions following seed implantation, including fever, hemorrhage, myelosuppression, skin and mucosal reactions, and seed migration. Skin and mucosal reactions were evaluated in accordance with the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) radiation-induced toxicity grading criteria. carcinoembryonic antigen (CEA) and cancer antigen 153 (CA153) levels were compared before and after treatment. These markers were assayed using chemiluminescence, with reference ranges of 0-5 ng/ml and 0-15 U/ml, respectively.
Lymphedema was classified into three grades based on the criteria of the International Society of Lymphology [8]: Grade I: Pitting edema that resolves with limb elevation and is nearly reversible spontaneously, with little to no subcutaneous fibrosis. Grade II: Persistent edema (not self-resolving) accompanied by subcutaneous fibrosis, presenting with a sensation of swelling, non-pitting edema, skin changes (e.g., hair loss), and nail alterations. Grade III: Tissue thickening due to lymph stasis, with the formation of keratotic, wart-like lesions on the skin.
Statistical analysis
Data analysis was performed using IBM SPSS 20.0 software. Quantitative data with a normal distribution were expressed as mean ± standard deviation (x ± s) and analyzed using the independent samples t-test or Wilcoxon signed-rank test. Qualitative data were presented as frequencies (percentages) and analyzed using the chi-square (χ2) test. Patient characteristics were described using proportions, medians, and ranges. Overall survival and LPFS were analyzed using the Kaplan-Meier method. Differences between groups were tested for significance using the log-rank test, and multivariate analysis using the Cox proportional hazards model was employed to assess the factors affecting survival. A p-value less than 0.05 was deemed statistically significant.
Results
Evaluation of efficacy
A total of 68 patients were included, with ages ranging from 36 to 82 years at the time of recurrence (median age: 56 years). Among them, 41 were postmenopausal and 27 were premenopausal. There were 89 locally recurrent lesions in total, with 77.94% (53/68) of patients presenting with solitary lesions. The distribution of lesions was as follows: 29.41% (20/68) were confined to the chest wall, 4.41% (3/68) to the supraclavicular lymph nodes, 17.65% (12/68) to the axillary lymph nodes, and 26.47% (18/68) to the breast. Additionally, 22.06% (15/68) of patients had multiple lesions (Table 2).
Table 2
Clinical features of locally recurrent metastases
The success rate of 125I seed implantation was 97.75% (87/89). The mean number of seeds implanted per lesion was 20.5 ±14.8, with a range of 11-50. The maximum diameter of locally recurrent lesions decreased from (5.69 ±2.74) cm before 125I seed implantation to (3.97 ±3.62) cm after the procedure, and the difference was statistically significant (t = 3.124, p = 0.0022 < 0.01). Six months after the procedure, 60 out of 68 patients had received chemotherapy, endocrine therapy, or targeted drug therapy. Tumor responses were evaluated after repeated clinical examinations, ultrasound, and CT scans of the 89 lesions. According to the efficacy evaluation criteria, there were 22 cases of CR, 45 of PR, 12 of SD, and 10 of PD. The LCR was 88.76% (79/89), and the ORR was 75.28% (67/89) (Figure 2).
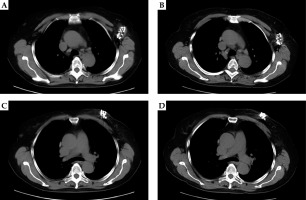
Fig. 2
Patient female, 65 years old, left breast invasive ductal carcinoma with local recurrence and left axillary lymph node metastasis. A) Left axillary lymph node metastasis after radioactive 125I seed implantation (preoperative lesion size about 4.05 cm × 2.78 cm). B) Left axillary lymph node metastasis 2 months after surgery (2.91 cm × 2.17 cm), evaluation PR. C) Image of the local recurrent focus of the left breast cancer after implantation of 125I seeds (the size of the preoperative lesion is about 2.65 cm × 2.15 cm). D) The local recurrent focus of left breast cancer was reexamined 2 months after surgery (2.28 cm × 1.48 cm), evaluating PR
Subgroup analysis of efficacy based on initial clinical data, treatment status, and local recurrence characteristics
Efficacy of initial clinical staging in 68 patients
Further subgroup analysis revealed the following results based on initial clinical staging: For patients with stage I + II, the LCR was 88.00% (22/25), and the ORR was 80.00% (20/25). For patients with stage III + IV, the LCR was 81.40% (35/43), and the ORR was 67.44% (29/43). Statistical analysis showed no significant difference in LCR between the two groups (χ2 = 0.472, p = 0.216 > 0.05). In contrast, the difference in ORR was statistically significant (χ2 = 9.822, p < 0.001).
Efficacy of monotherapy and systemic therapy in 68 patients
The ORR and LCR were 75.00% and 86.67%, respectively, in the 60 patients who received systemic therapy, compared with 50.00% and 62.50% in the 8 patients who received monotherapy. Statistical analysis showed that the differences in both ORR and LCR between the two groups were statistically significant (ORR: χ2 = 9.262, p < 0.001; LCR: χ2 = 10.196, p < 0.001).
Efficacy of local regional recurrence sites in 68 patients
The ORR and LCR were 60.00% and 73.33%, respectively, in 15 patients with multi-site recurrence, compared with 75.47% and 86.79% in 53 patients with single-site recurrence; the differences were statistically significant (χ2 = 8.215, 4.662, p < 0.001).
Relief of clinical symptoms
All 68 patients presented with pain. At the end of treatment, 50 cases achieved pain relief, resulting in a pain relief effective rate of 73.53%. Pain was re-evaluated 1 month after the procedure: 62 patients experienced pain relief, with the effective rate increasing to 91.18%. Additionally, 2 patients reported aggravated pain compared to the previous assessment, and 4 patients had no pain relief. Notably, one patient with aggravated pain was newly diagnosed with axillary lymph node metastasis.
Among these patients, 6 cases developed upper limb edema due to axillary metastasis, including 2 cases of grade I and 4 cases of grade II. After treatment, the edema regressed to varying degrees: 2 cases achieved complete resolution, 3 cases improved to grade I, and 1 case remained at grade II.
Serum tumor markers
The comparison of serum CEA and CA153 levels before and after treatment is presented in Figure 3 and Table 3. Statistical analysis revealed that the differences in both markers before and after treatment were statistically significant (CEA: t = 2.636, p < 0.05; CA153: t = 2.087, p < 0.05).
Fig. 3
A) Comparison of CEA before and after treatment (x ±s). The horizontal axis represents before and after treatment, and the vertical axis represents the levels of CEA; * indicates that the difference in the levels of CEA before and after treatment is significant (t = 2.636, p = 0.0094). B) Comparison of CA153 before and after treatment (x ±s). The horizontal axis represents before and after treatment, and the vertical axis represents the levels of CA153; # indicates that the difference in the levels of CA153 before and after treatment is statistically significant (t = 2.087, p = 0.0388)
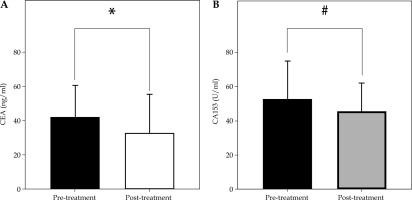
Table 3
Adverse reactions associated with 125I seed implantation therapy
Treatment-related adverse events
During 125I seed implantation, the most common adverse reaction was transient exacerbation of pain, observed in 16 patients (23.53%). This was attributed to local tissue swelling and compression of surrounding structures following lesion puncture. Local numbness occurred in 9 patients (13.24%); in these cases, discomfort was alleviated by slight withdrawal or repositioning of the needle, with supplemental local anesthetic administered as needed.
After 125I seed implantation, most adverse reactions were grade 1 or 2, with an overall incidence of 36.96%. No adverse reactions of grade 3 or higher were noted. Specifically, 19 patients (27.94%) developed low-grade fever, with body temperatures ranging from 37.5°C to 38.6°C. Twelve patients with low-grade fever returned to normal after physical cooling, while 7 improved with symptomatic treatment. Additionally, 6 patients (8.82%) developed localized hard nodules after treatment, which were attributed to residual fibrous tissue and retained seeds; these nodules improved spontaneously without special intervention. Local skin pigmentation (characterized by dark, rough skin without ulceration) was observed in 9 patients (13.24%). Of these, 7 cases improved spontaneously, and 2 showed gradual improvement after local treatment. Acute nausea was observed in 2 patients (2.94%), which improved with symptomatic treatment. Seed migration occurred in 1 patient (1.47%), who had a large localized ulcer at the site of chest wall recurrence. Notably, no severe complications were observed in the entire cohort, including hemorrhage, needle tract implantation metastasis, pneumonia, pulmonary radiofibrosis, exacerbated upper extremity or breast edema, or nerve injury (Table 4).
Long-term results
As of May 31, 2024, all 68 patients had completed follow-up without loss to follow-up. The follow-up duration ranged from 13 to 54 months, with a median of 32 months. By the end of the follow-up period, 40 patients (58.82%) had died, categorized as follows: 14 cases (20.59%) of non-cancer-related deaths, 19 cases (27.94%) of deaths due to systemic tumor metastasis, and 7 cases (10.29%) of deaths from multi-organ failure. The LPFS time was 25.60 months (95% CI: 20.36-26.70). The 1-year, 2-year, and 3-year LPFS rates were 82.35%, 52.94%, and 17.65%, respectively. The OS time was 36.65 months (95% CI: 34.50-40.20). The 1-year, 2-year, and 3-year OS rates were 95.59%, 70.59%, and 51.47%, respectively. In univariate and multivariate analyses, primary tumor stage, molecular subtype, treatment modality, and recurrence site were identified as independent factors influencing survival in patients with locally recurrent breast cancer (p < 0.05) (Table 5).
Table 5
Multivariate risk factor analysis of 125I seed implantation and local recurrence of cervical cancer
Comparison of survival in patients by initial clinical staging
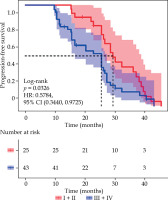
The LPFS time was 28.60 months (95% CI: 25.60-36.20) in patients with initial clinical stage I + II, compared with 20.36 months (95% CI: 15.40-25.60) in those with stage III + IV. The difference in LPFS between the two groups was statistically significant (χ2 = 4.567, p = 0.0326) (Figure 4).
Fig. 4
Kaplan-Meier curves of localized progression-free survival for initial clinical staging, comparison of survival curves for patients with stage I + II (n = 25) and stage III + IV (n = 43) (χ2 = 4.567, p = 0.0326)
The OS time was 47.5 months (95% CI: 42.30-51.60) in patients with stage I + II, compared with 30.80 months (95% CI: 23.20-35.60) in those with stage III + IV. The difference was statistically significant (χ2 = 7.739, p = 0.0054).
Comparison of survival in patients by different molecular typing
The LPFS times for patients with different molecular subtypes were as follows: 25.95 months (95% CI: 19.20-30.20) for Luminal A, 25.60 months (95% CI: 17.20-30.20) for Luminal B, 20.36 months (95% CI: 11.50-28.60) for HER-2 positive, and 11.55 months (95% CI: 9.80-26.30) for triple-negative. The difference in LPFS among the four subtypes was statistically significant (χ2 = 4.068, p = 0.0437) (Figure 5).
Fig. 5
Kaplan-Meier curves for molecularly typed local progression-free survival, Luminal A (26), Luminal B (25), HER-2 positive (11) and triple negative (6) patients were compared (χ2 = 4.068, p = 0.0437)
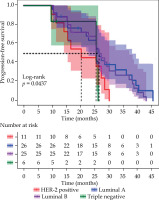
The OS times stratified by molecular subtypes were as follows: 41.25 months (95% CI: 38.05-46.70) for Luminal A, 34.80 months (95% CI: 22.80-46.20) for Luminal B, 35.60 months (95% CI: 22.90-46.80) for HER-2 positive, and 20.15 months (95% CI: 11.60-23.20) for triple-negative subtypes. Statistical analysis showed a significant difference in OS among the four subtypes (χ2 = 8.942, p = 0.0301).
Comparison of survival between monotherapy and systemic therapy patients
The LPFS time was 25.60 months (95% CI: 20.36-28.60) in 60 patients who received systemic therapy following 125I seed implantation, compared with 13.55 months (95% CI: 9.80-26.30) in 8 patients who underwent only 125I seed monotherapy. A statistically significant difference in LPFS was observed between the two groups (χ2 = 9.170, p = 0.0025) (Figure 6).
Fig. 6
Kaplan-Meier curves of local progression-free survival for different treatment regimens, comparing survival curves of patients treated with systemic therapy (n = 60) with those treated with single therapy (n = 8) (χ2 = 9.170, p = 0.0025)
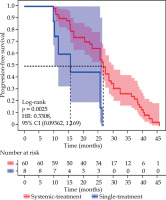
The two OS times were 38.23 (95% CI: 34.80-45.60) months and 22.85 (95% CI: 11.00-38.40) months, respectively, with a statistically significant difference (χ2 = 9.317, p = 0.0023).
Comparison of survival in patients with localized regional multisite recurrence vs. single-site recurrence
The LPFS time was 15.60 months (95% CI: 13.20-26.70) in 15 patients with multi-site recurrence, compared with 25.60 months (95% CI: 22.90-28.60) in 53 patients with single-site recurrence. There was a statistically significant difference in LPFS between the two groups (χ2 = 4.060, p = 0.0439) (Figure 7).
Fig. 7
Kaplan-Meier curves of local progression-free survival for different sites of recurrence, comparing the survival curves of patients with multiple sites of recurrence (n = 15) and single site of recurrence (n = 53) (χ2 = 4.060, p = 0.0439)
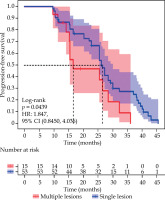
The two OS times were 34.90 (95% CI: 20.20-39.20) and 38.05 (95% CI: 34.50-46.62) months, respectively, and the difference was statistically significant (χ2 = 4.272, p = 0.0388).
Discussion
Local recurrence in the chest wall or regional lymph nodes (including axillary, supraclavicular, or intramammary nodes) occurs in approximately 10% to 40% of breast cancer patients after initial treatment [9], with chest wall recurrence being the most prevalent form of locoregional recurrence. Clinically, it typically manifests as one or more asymptomatic cutaneous or subcutaneous nodules on the chest wall, which are either within or adjacent to the surgical scar. Accounting for 50.0% to 94.0% of all localized relapses, breast cancer local recurrence is associated with a disease-specific mortality rate of 15% and a low rate of re-control after recurrence. Postoperative local recurrence of breast cancer is a potential precursor to distant metastasis, which occurs in approximately 60.8% to 80.0% of patients and often leads to death [10, 11]. In our cohort, there were 68 patients with first-time breast cancer recurrence, among whom 26 cases (38.24%) presented with chest wall recurrence. Local recurrent tumors tend to infiltrate the chest wall and skin, primarily due to the thin chest wall tissue and anatomical alterations following breast cancer surgery. While smaller recurrent lesions can be resected surgically, moderately larger ones are often deemed inoperable due to dermatological constraints (e.g., inadequate skin coverage or extensive cutaneous involvement). Additionally, supraclavicular lymph node metastases frequently present as fused masses, further complicating surgical intervention. Currently, radiation therapy is recommended for postoperative breast cancer patients with axillary lymph node metastasis, tumors located in the inner breast quadrant, or those who underwent breast-conserving surgery, with the aim of reducing local recurrence. Data from the National Surgical Adjuvant Breast and Bowel Project (NSABP), an international collaborative study, show that the 5-year recurrence rate after radical mastectomy alone (20.9%) is twice as high as that in patients who received radiation therapy following mastectomy (10.4%). Postoperative radiotherapy plays a crucial role in breast cancer management; however, re-irradiation is often challenging for patients with local recurrence, primarily due to radiation-induced side effects and extensive damage to surrounding tissues.
In recent years, 125I radioactive seed brachytherapy has been used clinically for the treatment of various solid malignant tumors both domestically and internationally. Due to its ability to be directly implanted into tumor tissue for localized, “zero-distance” tumor cell killing, 125I seeds are sometimes referred to as an “in vivo gamma knife”. The clinical efficacy of 125I seeds is mediated by γ-ray-induced tumor cell destruction. Its biological mechanism primarily involves the direct effects of radiation on DNA molecular chains, including both single-strand and double-strand breaks, which render tumor cells incapable of reproduction. This is followed by an indirect effect: γ-rays ionize water molecules in the organism, generating free radicals (with a lifespan of only 5-10 seconds) that interact with biological macromolecules, thereby causing damage to tissue cells. Permanent implantation of 125I seeds represents a highly effective local treatment modality for tumors, with distinct advantages: precise targeting of the lesion, minimal or no bleeding, continuous low-dose-rate irradiation (with an effective duration of 180-240 days) that ensures high therapeutic efficacy, full utilization of radiation energy, minimal damage to normal tissues, and no pain for patients. When combined directly with surgery and radiotherapy, it can reduce the surgical scope, improve the tumor cure rate, and lower the recurrence risk. Compared with other tumor treatment modalities, it offers advantages such as minimal trauma and improved quality of life. In contrast to external beam radiotherapy, its radiation source is miniaturized and hermetically sealed, thus posing no radiation hazard to surrounding medical staff or patients. Additionally, it can reduce the total body irradiation dose, decrease the need for chemotherapy, and mitigate surgical complications [12]. No grade 3 or higher adverse events were observed in the patients in this study. Grade 1 and 2 adverse events were primarily mild reactions, such as low-grade fever and localized skin pigmentation, which did not impair patients’ quality of life. Notably, seed detachment occurred in one patient with locally recurrent breast cancer of the chest wall, whose lesion was accompanied by a large ulcer. During hospitalization, the patient received local treatment in consultation with the Department of Dermatology; six months later, the large local ulcer had improved significantly. These findings indicate that the incidence of adverse events associated with radioactive seed implantation is significantly lower than that of external beam radiotherapy.
Yi et al. [13] reported on 43 patients with locally recurrent or metastatic breast cancer, all of whom developed local recurrence or metastasis after undergoing systemic treatments including surgery, chemotherapy, and radiotherapy. Most of their target lesions showed gradual shrinkage around 3-4 weeks after 125I seed implantation, with an overall effective rate of treatment of 96.3%, and a local control rate of 85.2% at 1 year. The evaluation of the long-term efficacy of the treatment in their study showed that, from the time of the appearance of recurrent metastatic lesions, the 1-year, 3-year, and 5-year patient survival rates after radioactive seeds implantation and systemic of other treatments were 95.3%, 67.4%, and 37.2%, respectively. In contrast to their study, our research enrolled patients who developed localized regional recurrence after initial surgery and radiotherapy and were unable to tolerate further surgery or radiotherapy. A total of 89 unresectable locoregional recurrent lesions in 68 breast cancer patients were treated with 125I radioactive seed brachytherapy in our study, achieving an implantation success rate of 97.75% (87/89). The maximum diameter of the target lesions decreased from 5.69 ±2.74 cm preoperatively to 3.97 ±3.62 cm postoperatively, with a statistically significant difference (p = 0.0022). Six months after postoperative systemic therapy, tumor responses were evaluated for all 89 lesions through repeated clinical assessments, ultrasound, and CT examinations. The local control rate (LCR) was 88.76% (79/89), and the overall response rate (ORR) was 75.28% (67/89). Despite differences in the selected patient cohorts, both the study by Yi et al. and our current research achieved initially satisfactory outcomes.
Analysis of the relationship between clinicopathological factors and survival in locally recurrent breast cancer revealed statistically significant differences in local control rates among subgroups stratified by primary tumor stage, recurrence site, and clinical staging. These findings suggest that patients with advanced primary disease, multiple recurrence sites, or triple-negative breast cancer have poorer LPFS. In contrast, the pathological type of the tumor did not significantly affect survival in locally recurrent breast cancer. Breast cancer exhibits high heterogeneity, and its diverse clinical and biological manifestations are determined by distinct genetic phenotypes.
Breast cancer can be categorized into Luminal A, Luminal B, human epidermal growth factor receptor 2 (HER2)-overexpressing, basal-like, and non-basal-like subtypes based on gene expression clustering analysis [14]. In clinical practice, it is classified into four subtypes – Luminal A, Luminal B, HER2-positive, and triple-negative – according to the expression levels of estrogen receptor (ER), progesterone receptor (PR), and HER2 detected by immunohistochemistry. Studies have indicated that, in addition to traditional classification approaches such as clinical and pathological features, molecular typing provides more evidence for treatment selection and prognostic prediction [15, 16]. In general, Luminal A subtype has the most favorable prognosis, while triple-negative subtype has the poorest [17, 18]. Consistent with this, our study also indicated that triple-negative breast cancer had poor survival, with triple-negative LPFS time of 11.55 months and OS time of 20.15 months, both of which were significantly shorter than the survival times of the other three molecular subtypes. Systemic therapy administered following locoregional recurrence of breast cancer has a statistically significant impact on both LPFS and OS. This suggests that systemic therapy may reduce the rate of distant metastasis and thereby improve patients’ OS.
The results of this study demonstrated that the pain relief rate at 1 month postoperatively was 91.18%, with a significant reduction in pain levels compared to those before treatment. Additionally, serum levels of CEA and CA153 were significantly lower than preoperative levels, with statistically significant differences (p < 0.05). These findings indicate that CT-guided radioactive seed implantation for the treatment of patients with localized recurrent breast cancer can reduce serum CEA and CA153 levels, confirming its accurate therapeutic efficacy. This can be attributed to the fact that 125I radioactive seed implantation exerts a direct cytotoxic effect on breast cancer cells, while also inhibiting tumor cell proliferation and accelerating their apoptosis [8]. The most widely used tumor markers for breast cancer are CA153 and CEA. CA153 is a high-molecular-weight glycoprotein with a relative molecular mass of 4,000,000, classified as a glycan antigen localized on the surface of tumor cells. When cells undergo malignant transformation, the activity of proteases and sialidases on the cell membrane increases, disrupting the cytoskeleton. This leads to the shedding of surface antigens, which in turn results in elevated serum CA153 levels. CA153 levels are elevated in 30-50% of breast cancer patients, and this proportion can reach as high as 80% in those with recurrent or metastatic disease [19].
This study provides robust evidence for the application of 125I seed brachytherapy in patients with unresectable locoregional recurrent breast cancer. Notably, some of the included patients with locoregional breast cancer recurrence had previously received treatments such as surgery, adjuvant chemotherapy, hormonal therapy, or EBRT. To investigate whether the efficacy of 125I seed brachytherapy varies based on prior treatment history, two subgroups will be established for efficacy comparison: 1) patients who underwent surgery combined with sequential postoperative treatment, 2) patients who did not receive surgery. The prognosis and survival outcomes of breast cancer patients with localized regional recurrent lesions treated with 125I seed brachytherapy across different recurrence risk groups have not been addressed. To resolve these issues, we will continue to collect a large sample of cases of localized recurrent breast cancer and systematically analyze the factors influencing the spatial distribution of radioactive seeds, as well as their correlations.
In summary, 125I radioactive seed implantation is an effective treatment for locoregional recurrent breast cancer lesions, characterized by minimal trauma and few complications. It thus serves as an important therapeutic option for locoregional breast cancer recurrence. This study indicates that advanced pathological staging, multisite recurrence, and triple-negative breast cancer are associated with poor local control and a high tendency for distant metastasis. Conversely, favorable prognostic factors include combination with systemic therapy, early primary tumor staging, and Luminal subtype, among others. Therefore, for locoregionally recurrent breast cancer, a multidisciplinary collaborative approach should be adopted to fully leverage the advantages of combined systemic therapy. Individualized treatment plans should be formulated based on the patient’s specific conditions, so as to maximize the efficacy of 125I radioactive seed implantation therapy.