Purpose
Gynecological cancers are a major cancer burden in India. Cancers of the uterine cervix, endometrium and vagina account for 110,000 cases annually, and cause 10% of all cancer deaths [1]. Subtotal or simple hysterectomies usually without pelvic nodal dissection, in the presence of an occult cervical or endometrial cancer, are not a rare occurrence [2-4]. The cancer in these cases most commonly recurs as an inoperable pelvic mass. Treatment comprises of radical radiotherapy with or without concurrent chemotherapy [5]. Optimal outcomes with radiotherapy require the use of external beam radiotherapy followed by brachytherapy to the residual disease. Brachytherapy may be delivered with vaginal cylinders and/or in the form of interstitial techniques with perineal templates. Interstitial brachytherapy allows more precise treatment of the disease, providing more uniform dose distribution over a larger area [6]. Furthermore, template-based implantation allows for good geometry with a fixed relationship of needles with target tissue and surrounding organs at risk [7, 8].
In our previous experience [9], we have reported outcomes of 113 patients with vault and vaginal cancers, treated with interstitial brachytherapy using Martinez universal perineal interstitial template (MUPIT) between January, 2000 and December, 2008. Meanwhile, concomitant chemo-radiation and progress in brachytherapy processes were systematically implemented in routine clinical practice. With the aim to study outcomes in a similar group of patients treated between January, 2009 to December, 2015, and to perform qualitative comparison with the previous published series, we undertook these analyses, which were the basis of this report.
Material and methods
After obtaining institutional ethics committee approval, post-hysterectomy recurrent gynecological cancer patients treated with external beam radiation (EBRT) with/without concomitant chemotherapy, followed by an high-dose-rate (HDR) interstitial brachytherapy boost using MUPIT between January, 2009 and December, 2015 at a tertiary cancer care hospital in India were analyzed. Patients enrolled in prospective clinical trials (NCT01391065, NCT01117402), and those who underwent re-irradiation with MUPIT-based HDR brachytherapy were excluded. Patient, disease, and treatment characteristics were noted. Patients were classified as those with primary vault/vaginal cancers, and those with post-operative recurrent cancers (either cervical or endometrial). Patient-related details, including age, comorbidity status, and hemoglobin level were documented. Disease-related details, such as histology, clinical tumor size, extent of vaginal and parametrial involvement, presence of hydroureteronephrosis, pelvic and para-aortic involvement, and presence of bladder or rectal involvement were all noted. Treatment details, including EBRT energy, field arrangements, doses and brachytherapy details, and dose and overall treatment time were obtained. Total radiotherapy dose was calculated in terms of equivalent dose in 2 Gy per fraction (EQD2).
External beam radiation and brachytherapy details with MUPIT procedure were the same as described in our previous publication [9]. All patients received EBRT to whole pelvis, followed by fractionated template-based interstitial brachytherapy. Median dose of EBRT was 50 Gy. Patients fit for chemotherapy received weekly concomitant cisplatin at 40 mg/m2 through the duration of EBRT. Median cycles of chemotherapy were 4 (range, 3-6 cycles). EBRT was followed by brachytherapy with MUPIT in 4-5 fractions of 4 Gy each, delivered twice daily over two to three days, using a single application. Response to EBRT was documented by examination under anesthesia at the time of implant insertion. Absence of gross disease on inspection by speculum and by bi-digital palpation was defined as ‘complete response’, and presence of any gross disease locally was specified as ‘partial response’. These findings were compared with the local examination at baseline; however, no patient experienced disease progression during EBRT. Silver markers were inserted prior to template implantation to define extent of the residual disease at brachytherapy. All patients underwent computed tomography (CT) imaging for brachytherapy planning. The brachytherapy planning principles included catheter reconstruction, source loading according to the desired treatment volume defined by placement of needles/tubes and silver markers, basic Paris system rules for interstitial brachytherapy, geometric optimization, and evaluation of doses to the rectum, bladder, and sigmoid. Total doses from EBRT and brachytherapy protocol were similar to those reported earlier. Major difference in this cohort was that the brachytherapy planning was CT-based, with contouring of the bladder, rectum, and sigmoid. However, the target was not delineated due to poor visualization of the disease, resulting from artefacts created by steel needles of the implant. Silver markers were applied as surrogates to ensure adequate coverage of the target.
Trans-rectal ultrasound was used to gauge the needle path and prevent rectal and bladder perforation. In rare instances, where the needles perforated the bowel, rectum, or bladder, those needles were not loaded. Such patients were monitored with abdominal girth charting and started on prophylactic antibiotics. Dose to organs at risk was controlled by using dwell-time as well as graphical optimization, to keep the 85% isodose line outside the critical structures. Needles that inadvertently passed through the bladder or rectal wall were not loaded. Brachytherapy was performed as a single-implant for 4 to 5 fractions, delivered twice daily, at least 6 hours apart. After completion of treatment, patients were followed up at regular intervals, 6 weeks post-treatment, followed by 3 monthly follow-up visits for the first two years, sixth monthly follow-up till five years post-treatment, and annually thereafter. Patients were assessed with physical examination at every follow-up visit, and additional testing was mandated only in case of specific symptoms or signs. Late toxicities were scored according to the Radiation Therapy Oncology Group criteria for the genitourinary and gastro-intestinal systems. Patients, who were not on regular follow-up were contacted over the phone to know their health status, and requested to physically attend follow-up visits in the hospital.
Statistics
Statistical analysis was performed with SPSS version 21 software (SPSS Inc., Chicago, IL, USA). Survival analysis was completed using Kaplan-Meier curves. Time to event analyses were done from the date of diagnosis, and patients lost to follow-up were censored on the date of last follow-up. Patients with recurrence in the vagina or in the vault within the irradiated volume of brachytherapy were considered as a local recurrence, while a recurrence within the pelvis, but outside brachytherapy-irradiated volume or pelvic nodal recurrence was considered as a regional recurrence. Local recurrence-free survival (LRFS) was calculated from the date of diagnosis to the date of local recurrence, while disease-free survival (DFS) was assessed from the date of diagnosis to the date of any recurrence, and overall survival (OS) was calculated till patient’s death due to any cause.
Patient, disease, and treatment-related factors, with the potential to affect outcomes, were converted to dichotomous variables, and univariate analysis was carried out using log-rank test for evaluating the impact of these factors on the outcome. As part of a secondary objective, results of the 117 patients analyzed in this study were compared to our previously published results of 113 patients treated with MUPIT interstitial brachytherapy. Patient, disease, and treatment characteristics, along with disease outcome and toxicity of the previously analyzed patients, were retrieved and qualitatively compared.
Results
Between January, 2009 and December, 2015, 271 wom-en underwent an interstitial brachytherapy boost using the MUPIT after EBRT for gynecological cancers at our institution. Eighty patients, who were enrolled in prospective clinical trials, and twenty-one patients, who underwent re-irradiation were excluded. Of the remaining 170 women, 53 patients’ medical records were missing; therefore, 117 patients were eligible for final analyses.
Patient, disease, and treatment characteristics are depicted in Table 1. Primary vault and vaginal cancers (patients with primary vaginal cancers, or post-hysterectomy due to non-oncological indications patients) accounted for 32.5% of all the cases, while recurrent vault cancers (post-hysterectomy due to oncological indications patients) accounted for 67.5% of the cases. The median age was 50 years (range, 28-76 years). Comorbidities, including hypertension, diabetes mellitus, or hypertension, were present in 12% of patients, and the mean hemoglobin concentration was 11.6 g/dl (range, 6.90-14.5 g/dl). Squamous cell carcinoma was the most common histology. 40% of the patients had bulky disease at presentation (clinical tumor size > 4 cm). All the patients received EBRT to the pelvis, with a median dose of 50 Gy (range, 42-50 Gy), and 65% of the patients received concurrent chemotherapy. On clinical examination, 22% of the cases had complete response to EBRT, while 78% had a partial response. No patient progressed during EBRT. The most used brachytherapy dose fractionation was 4 Gy × 5 fractions, and the total median dose received by the patients was 73.3 Gy EQD2 (range, 63-78 Gy EQD2). The median overall treatment time was 63 days (interquartile range [IQR], 53-72 days). Out of the 117 patients, 88 had OTT of more than 56 days. The median follow-up of the surviving patients was 63 months (IQR, 33-88 months). Table 2 shows pattern of the first recurrence after radiotherapy. At the time of last follow-up, 55 patients had relapsed, with 31 (25.4%) patients experiencing local recurrence component with/or without distant/regional failures, and 23 (19.6%) patients having distant failure with/without local/loco-regional failures; 62 patients were disease-free.
Table 1
Patient, disease, and treatment characteristics
Table 2
Pattern of first relapse
| Pattern of first relapse | Number (%) |
|---|---|
| Local | 15 (12.8) |
| Loco-regional | 8 (6.8) |
| Distant | 15 (12.8) |
| Local/loco-regional + distant | 8 (6.8) |
| Not known | 9 (7.7) |
Survivals
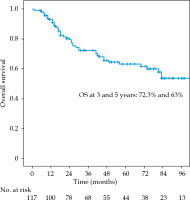
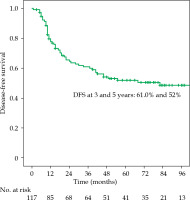
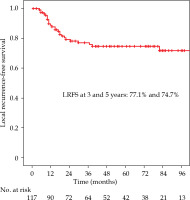
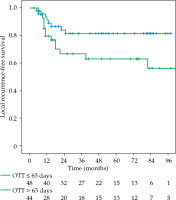
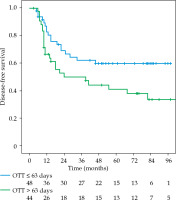
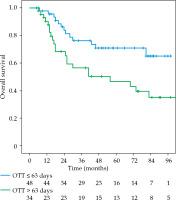
The local recurrence-free survival, DFS, and OS for the whole group at 3 and 5 years were 77.1%/74.7%, 61%/52%, and 72.3%/63%, respectively (Figures 1-3). Cervical tumor diameter more than 4 cm has been implicated with inferior disease-free and overall survival in cancer of the intact cervix [10, 11]. Therefore, a cut-off value of 4 cm at presentation was accepted to define bulky disease and assess its’ impact on the outcomes. Patients with bulky disease at the presentation (clinical tumor size > 4 cm), and those with residual disease at brachytherapy presented worse LRFS (p = 0.01 and p = 0.06, respectively). Overall treatment time of more than 56 days did not significantly affect LRFS and DFS, with a tendency towards improvement in OS (p = 0.072). OTT of more than 63 days (median OTT) adversely impacted LRFS (p = 0.04), DFS (p = 0.04), and OS (p = 0.01) (Figures 4-6). Table 3 demonstrates the results of univariate analysis.
Table 3
Univariate analysis
Toxicities
Acute toxicities were not documented in the present study. The overall grade 2-4 late toxicities were observed in 19 patients, urinary toxicities in 4 (3.4%), and gastro-intestinal (rectum and bowel) in 16 patients (13.6%); one patient presented with both urinary and gastro-intestinal toxicities. Bladder toxicities in the form of grade 2/3/4 cystitis were seen in 2/1/1 patients, respectively. One patient with grade 4 cystitis underwent angio-embolization of the superior vesical artery, while the patients with grade 2 and 4 cystitis were managed conservatively with cold saline irrigation and cystoscopic evacuation of clots. In the patients with gastro-intestinal toxicity (rectal and small bowel) seen in 16 (13.5%) patients, grade 2/3/4 proctitis were seen in 7/5/1 patients, respectively, and small bowel obstruction in 3 patients. Patients with radiation proctitis were managed with laxatives, steroid enemas, and argon plasma coagulation. On resolution of the acute bleeding episode, patients also received hyperbaric oxygen therapy, if appropriate. Three patients, who developed late intestinal obstruction that were attributed to radiation, underwent surgery for the same reason.
As a part of our secondary objective, we compared the results of the present analysis with our previous reported series [9]. Table 4 shows comparison of patients, diseases, treatments and outcomes characteristics between the two cohorts treated in different time periods. The patient and disease characteristics were similar in both the groups. Brachytherapy dose and fractionation remained the same. In the present cohort, there was an increase in concomitant chemotherapy use (65% vs. 35%). With a longer median follow-up of 63 months in the present cohort, the outcome is better in terms of local control and overall survival as well as a decrease in the incidence of late bladder and gastro-intestinal toxicities, but with an increase in distant metastases rates. Moreover, in 7.7% of patients, the relapse rates were not documented, which could be considered as disease-free.
Table 4
Comparison between old and new studies
Discussion
Inadvertent simple hysterectomies in the setting of occult gynecological cancers is not an uncommon occurrence in our patients’ population [2, 12]. Brachytherapy in the setting of recurrent or vaginal cancers is challenging due to the distorted anatomy and incomplete coverage with standard applicators [13]. Some of these challenges can be overcome with interstitial brachytherapy [6, 14]. In addition, template brachytherapy allows better homogeneity, particularly in larger implants [7].
Reported literature in the setting of vault and vaginal cancers is sparse, and mainly retrospective in nature. Our previous experience of 113 patients (2000-2008) treated using template-based HDR brachytherapy for the vault and vaginal cancers, is associated with reasonably good outcomes and acceptable toxicities [9]. With evolving treatment strategies, especially the use of concomitant chemotherapy in gynecological cancers, we intended to undertake an audit of patients with recurrent vault and vaginal cancers treated between 2009-2015, which formed the basis of the current report. The results of this cohort was also compared with the previous experience for better understanding in terms of disease outcome and toxicities.
Our present analysis included 117 patients with primary or recurrent vault and vaginal cancers. The median age of patients was 50 years, and squamous cell carcinoma was the most common histology. 32.5% of patients presented with primary vault or vaginal cancers, while 67.5% presented with vault recurrence. As shown in Table 4, patients and disease characteristics were comparable with our previous published series of patients treated between 2000-2008. The use of newer conformal external beam delivery techniques was higher in the current series. Major difference between the two studies was the use of concurrent chemotherapy; it was higher (65% vs. 35%) in the current series. This represents the implementation of the practice of concurrent chemoradiotherapy for locally advanced cervical cancer at our institution. The median follow-up was longer, i.e., 63 (IQR, 33-88) months vs. 43 (IQR, 19-67) months, with possibly better detection of the events in the current series. The 3-year DFS was similar, 60.5% and 61%, respectively, and the 3-year OS was 62.2 and 72.3%, respectively, in the old and new series. However, the disease-free survival was similar, and the pattern of local first recurrence occurred more frequently in the older series (34% vs. 26% in the old and present series, respectively); however, in 7.7% of patients, the relapses were unknown. This trend towards better local control is likely due to an increase utilization of concomitant chemotherapy (35% vs. 65%). The better overall survival may also be due to more aggressive salvage therapy in the current series, where radical re-irradiation was attempted in 3 patients, salvage surgery in 1 patient, and chemotherapy in 16 patients at first recurrences. Most importantly, a large number of patients were censored for overall survival at the date of diagnosis of recurrence in our previous series, with patients being referred to peripheral centers for salvage therapy. Better telephonic follow-up and the use of electronic medical records in the present series, may also have contributed to better computation of overall survival in the current study.
Additionally, there was a substantial reduction in symptomatic late (grade 3-4) toxicities from 10% to 5.1% for rectal toxicities, and from 4.4% to 1.7% for the bladder, respectively. The improvement in survival and reduction in late toxicities maybe attributed to an increase utilization of concomitant chemoradiation regimen, improvement and refinement in brachytherapy treatment, including utilization of CT imaging, optimization of doses to the rectum and bladder, and disease-specific dedicated team approach. This is also evident in the recent experience published using newer imaging (magnetic resonance imaging [MRI] and positron emission tomography [PET]) and radiation techniques in a prospective phase II study at our institution [15]. There was an improvement in LC, DFS, and OS compared with the previous study. This showed some correlation of D2cc rectal dose of 55 and 66 Gy EQD2 to be associated with the risk of rectal toxicity of 10% and 20%, respectively [16]. This approach may allow for better target delineation and dose optimization to improve the disease outcomes and toxicities further [15, 16]. Nevertheless, what we report here is the patients’ cohort evaluated and treated in routine clinical practice, which represents clinical outcomes outside of clinical trial settings. Recent data has shown that the use of MRI for brachytherapy planning in cervical cancer, allows dose escalation to the target and reduced dose to organs at risk, leading to a better local control. Similar benefit may be seen with the use of MRI during template-based brachytherapy with newer templates, which are MR-compatible [17-20]. Late urethral toxicity is a new field of investigation. Dose of 0.1 cc to the urethra has correlated with late grade 3 urinary toxicity in several retrospective studies [21-23]. There have also been some interesting studies in recent times regarding the use of template-based brachytherapy. Marar et al. developed a 3D-printed patient-specific template as the adjunct to a tandem and ovoid application in patients with cervical cancer. They showed that, compared with no-needle or free-hand needle approach, the use of template-guided needle insertion resulted in a higher mean V100% and D90 doses to the target [24]. The recently developed Kelowna GYN template (Varian Medical Systems, Palo Alto, CA, USA) is similar to the MUPIT, with additional benefit of being MR-compatible and allowing the use of plastic needles in addition to titanium. Shiao et al. have published early results of their successful use in various gynecological malignancies, with a similar patients’ population as ours [25].
The strengths of our study are the relatively larger patients’ cohort in this unique population of patients, well-represented patient groups, homogeneous dose of radiotherapy with better compliance to concomitant chemoradiation schedules, and longer follow-up period with clinical outcomes. The comparison of clinical outcomes between different time frames from a single-institution also reflects the need for the establishment of regular audits and systematic improvement in routine clinical practice [26, 27]. The limitations of our study include its’ retrospective nature, and lack of brachytherapy dosimetric data for correlation with clinical outcomes, including toxicities.
Conclusions
Patients with primary and recurrent vault and vaginal cancers treated with radical radiation therapy, including high-dose-rate interstitial brachytherapy boost, using MUPIT, resulted in modest clinical outcomes and acceptable late toxicities. As compared with our previously published series, the outcome of the current study is relatively better in terms of both clinical outcomes, basically due to concomitant chemoradiation and late toxicities due to CT-based optimization of doses to organs at risk.