Purpose
Bone metastases occur in approximately 80-90% of advanced prostate cancers [1, 2], which may lead to the development of bone-related events, such as intolerable bone pain, pathologic fractures, spinal cord compression, and hypercalcemia. Among these, bone pain is the earliest symptom that significantly reduces the quality of life of a patient, and pain relief is the main goal of palliative care. Pathophysiology of pain due to bone metastases is complex and still unknown; therefore, symptomatic treatment is based on non-specific therapy rather than complete eradication of the disease.
For focal metastases, external radiotherapy is the most effective local treatment for the relief of bone pain [3]. However, the prescribed dose of external radiotherapy may be less than optimal when pain relief is compromised in cases, in whom the lesion is adjacent to paraspinal cord, or where the margins are not clear with respect to vital organs. In patients with diffuse metastases or pain after receiving external radiotherapy, a combination of strontium chloride (89SrCl2) or bisphosphonates, hormonal therapy, and personalized doses of analgesics are used, but side effects can occur due to resistance or dosage, among others. Therefore, the physician must weigh the expected clinical benefits and risks when side effects of these therapies are considered in decision-making.
According to previous studies, 125I seed implantation is an acceptable, useful, and minimally invasive treatment method with high-rate of local control [4-6]. However, 125I brachytherapy is used to relieve bone metastasis-related pain of prostate cancer and unsuccessful treatment after conventional therapy. This study included 48 patients, who were treated at the Nuclear Medicine Department of the Northern Theater General Hospital. Patient data were retrospectively analyzed to better evaluate the feasibility and safety of radioactive 125I seed implantation brachytherapy combined with 89SrCl2 for relieving pain in patients with prostate cancer bone metastases after failure of external irradiation.
Material and methods
Research object
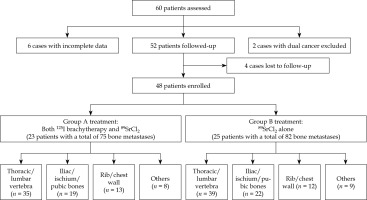
Clinical data of 60 patients with moderate to severe pain due to prostate cancer bone metastases, admitted to the Department of Nuclear Medicine of the General Hospital of Northern Theatre Command from January 2019 to December 2023 were retrospectively analyzed. After inclusion and exclusion criteria, a total of 48 patients were finally included and divided into two groups. In group A (n = 23), patients underwent radiological 125I seed implantation combined with 89SrCl2 treatment, while in group B (n = 25), patients underwent 89SrCl2 therapy alone (Figure 1). The study was approved by the Ethics Committee of the hospital (Ethics No. YL2021-07), and all patients signed informed consent form. Enrolled patients had been previously using endocrine therapy, molecular targeted therapy, or external irradiation for pain control, but did not achieve satisfactory pain relief. The general information of the patients are shown in Table 1. There was no significant difference between the two groups in clinical and tumor characteristic variables (p > 0.05).
Table 1
Clinical characteristics of patients and tumors
Inclusion criteria: 1. Prostate cancer confirmed by FNAC or post-operative pathology; 2. Bone metastasis was the only possible cause of pain; 3. The worst pain intensity score was at least 4 points during 24 hours prior to completing brief pain inventory (BPI) [7]; 4. Diameter of bone metastases ≤ 5 cm; 5. The expected survival period > 3 months; 6. Karnofsky performance scale (KPS) ≥ 50; 7. No serious coagulation disorders (thromboplastin activity < 40%, routine blood tests: WBC ≥ 4.0 × 109/l, RBC ≥ 3.5 × 1012/l, PLT ≥ 100 × 109/l, liver and kidney function tests within normal ranges). 8. High-dose analgesics utilization for more than 1 month (patient receiving an oral morphine equivalent dose (MEDD) of ≥ 60 mg per day for more than 1 month).
Previous treatment: All patients had received external irradiation treatment, of which 38 cases were localized recurrence after external irradiation treatment, and 10 cases were recurrence after 2 courses of external irradiation treatment. External beam radiotherapy was delivered using 6-MV X-rays with conventional fractionation, such as 200 cGy per fraction to a total dose of 4000 cGy in 20 fractions, or 300 cGy per fraction to a total dose of 3000 cGy in 10 fractions. Time interval between the last external irradiation treatment and seed implantation treatment was 8.7 ±2.4 months.
Exclusion criteria: 1. Other malignant tumors; 2. Cachexia; 3. Suffering from serious cardiovascular or mental diseases; 4. Incomplete clinical data or lost to follow-up.
Instruments and equipment
A 64-row CT conventional scanner Discovery VCT PET/CT (GE) for seed implantation scanning was employed. The required implantation gun and push thimble was produced by Xiangshan, Zhejiang, China, with 15-20 cm × 18 G puncture needle (Yaguang, Japan). Three-dimensional treatment planning system (TPS) for radiation implantation therapy was used (Beijing Feitian Zhaoye Technology Co., Ltd.). Seed source was produced and provided by Beijing Atomic High Tech Co., Ltd, with the seed size of 0.8 mm × 4.5 mm, sealed cylinder with titanium coating, half-life of 59.7 days, and average photon energy of 27~35 KeV. Seed activity was 29.6 MBq, matched peripheral dose (MPD) was 109.2-145.6 Gy, and planned target volume (PTV) was 1 cm outside clinical target volume (CTV). Prostate specific antigen (PSA) and free PSA (fPSA) were detected using chemiluminescence method, with Liaison XL automatic chemiluminescence immuno-analyzer (Soling Diagnostics, Co., Ltd., Italy); the kit was provided by the company, with reference range of 0-4 ng/ml and 0-1.5 ng/ml, respectively. ALP was evaluated by rate method using Beckman Coulter automatic biochemical analyzer, and reference range was 45-125 U/l.
125I seed implantation
Enhanced CT images within 1 week before treatment were imported into TPS to design the needle entry direction, number of seeds implanted, arrangement of puncture needles, etc. Laboratory tests were performed within 3 days pre-treatment, and the operation risk and safety were evaluated. Different body positions were selected according to the lesion location, external positioning grating was placed in the corresponding skin area of lesion, CT scanning was conducted to locate and select the puncture point, and needles was assembled layer-by-layer according to TPS plan. Implantation was performed under CT guidance with local anesthesia. During procedure, position and direction of the implanted needle were adjusted according to CT scan, so that the needle’s depth in the lesion was 0.5 cm from the tumor edge and through the tumor’s center. Patient vital signs were monitored throughout the surgery, with consciousness, pain, respiration, and other conditions observed. After the operation, the puncture point was pressed for 10-20 minutes and patient state was observed.
89SrCl2 internal irradiation therapy
All 48 patients received 89SrCl2 internal irradiation treatment, and relevant laboratory tests, including blood routine, liver and kidney function, coagulation, blood calcium, prostate cancer tumor markers, etc. were completed before the procedure. Strontium-89 (89Sr) injection (Chengdu CNC Gaotong Isotope Co., Ltd., state drug license: H20041312) was used for treatment. For patient with lower or heavier body mass, a 100-150 MBq (4 mCi)/dose was administered and calculated as 1.5-2.0 MBq (40-50 μCi)/kg. After drug unsealing, the required single-dose of 89SrCl2 was injected slowly and intravenously into the patient, over 1-2 minutes.
Follow-up and bone pain relief observation indicators
Until January 30, 2025, no patient was lost to follow-up. The median follow-up was 7.5 months (range, 5.5 to 15 months). Patients were followed-up at 3 days, 4 weeks, 8 weeks, and 12 weeks after treatment, and every 2 months thereafter. The visit included scales, imaging examinations, and laboratory tests. BPI scores (each ranging from 0 to 10) included pain level (worst pain, least pain, average pain, and present pain) scores and bone pain interference (daily activities, mood, ability to walk, daily work, social activities, quality of sleep, and interest in life) scores, with pain level scores ranging from no pain (0) to the strongest (10), and bone pain interference scores ranging from 0 to 10 (a higher score indicates a better quality of life). Patients completed the above scales pre-treatment, and at 3 days, 4 weeks, 8 weeks, and 12 weeks after treatment with the assistance of a specialized pain physician. Moreover, all patients underwent enhanced CT and hematological examinations (blood routine, liver and kidney functions, ions, etc.) within 3~5 weeks after the procedure to evaluate the safety and effectiveness of the treatment [8], PSA, fPSA, both with normal reference ranges of 0~4 and 0~1.5 μg/l, and alkaline phosphatase (ALP), with normal reference range of 45~125 U/l. Intra- and post-operative complications were recorded.
Statistical analysis
IBM SPSS version 20.0 software was used to analyze data. Quantitative data conforming to normal distribution were expressed as x ± s using two-sample independent t-test or Wilcoxon rank-sum test. Qualitative data were expressed as frequency (percentage) and analyzed by χ2 test. A four levels of pain scores before treatment, 3 days after treatment, 4 weeks after treatment, 8 weeks after treatment, and 12 weeks after treatment were analyzed using repeated-measures ANOVA, while four levels of pain scores of groups A and B were analyzed with Bonferroni method. P-value < 0.05 was considered as statistically significant difference.
Results
Seed implantation
Group A consisted of 23 patients with a total of 75 bone metastases, while Group B included 25 patients with 82 bone metastases. A total of 39 bone metastases in group A underwent 125I seed implantation (Figure 2), with 16 lesions having a maximum diameter of ≤ 2 cm, 17 lesions measuring between 2 cm and < 4 cm, and ≥ 6 lesions between 4 cm and ≤ 5 cm. Twenty lesions (51.28%, 20/39) were located in the cervical, thoracic, and lumbar vertebrae, 15 lesions (38.46%, 15/39) in the ilium, ischium, and pubis, and 4 lesions (10.26%, 4/39) in the scapula, ribs, and chest wall. The success rate of 125I seed implantation was 97.44% (38/39), with a total of 722 seeds implanted. Each lesion was implanted with 10-36 (18.5 ±7.0) seeds, and the total activity was 296-1,056.6 MBq. The total absorbed dose was 108.7-201.4 Gy.
Fig. 2
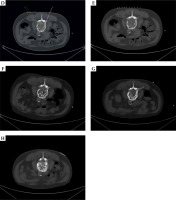
A) Whole-body bone imaging. A 72-year-old patient diagnosed with prostate cancer on puncture biopsy with a Gleason score of 8 was treated with conventional endocrine therapy. Three years later, whole-body bone imaging for bone pain revealed abnormal radionuclide concentrations at lesions in the thoracic 8 vertebrae, lumbar 2-4 vertebrae, and left iliac bone, suggesting multiple bone metastases. Therefore, radioactive 125I seeds at sides of bone metastases were implanted. B) Dose-volume histogram indicating prescribed dose to lesion and dose received by the spinal cord. C) Dose-volume histogram indicating prescribed dose to lesion and dose received by the spinal cord

Fig. 2
Cont. D) The irradiation dose (green line area) of 125I brachytherapy field, tumor contour (green line), and spinal cord (orange line). E) Pre-operative lesion size of third lumbar vertebra was about 4.72 cm × 3.73 cm. F) Radioactive 125I seeds were implanted into the metastatic lesion of third lumbar vertebra. G) 3 months post-treatment lesion review (4.01 cm × 3.32 cm). H) 6 months post-treatment lesion review (3.84 cm × 3.21 cm), overall evaluation PR
Surgery-related complications
In group A, fever was observed in 4 patients on the 2nd post-operative day, a small amount of bleeding from the puncture site was seen in 2 patients, and slight displacement of 125I seeds was detected in 2 patients after the tumor volume was reduced, with an incidence rate of 17.39%, 8.70%, and 8.70%, respectively. There were no serious complications of radiation injury, such as hemorrhage, skin breakdown pigmentation, or in vital organs. The surgery-related complications are shown in Table 2.
Table 2
125I brachytherapy-related complications
Bone pain relief
In Table 1, pain level scores are shown. Below, we present comparison within each group and comparison between the groups.
Comparison within each group
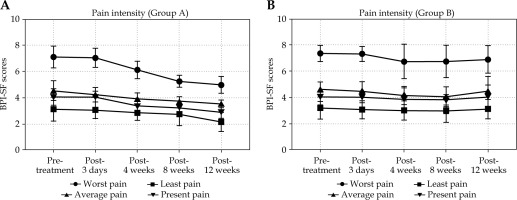
The worst pain, average pain, and present pain scores of group A decreased to varying degrees with time (F = 22.47, 5.22, 3.71; p < 0.001, 0.001, 0.007). In group A, the differences in the worst pain, average pain, present pain scores before and after treatment were statistically significant at 12 weeks after treatment (t = 6.14, 3.36, 2.86; p < 0.001, 0.002, 0.007). The worst pain at 3 days, 4 weeks, 8 weeks, and 12 weeks after treatment was not statistically significant compared with before treatment at 3 days after treatment (t = 2.01, p = 0.0501), while statistical significance was observed at the remaining 4-week, 8-week, and 12-week follow-ups (t = 7.11, 5.31, 6.14; both p < 0.0001). Furthermore, there were differences in the worst pain, average pain, and present pain scores of group B before and after treatment (F = 2.52, 2.45, 2.45; p = 0.044, 0.049, 0.049). The scores initially decreased (until 8 weeks after treatment, the differences in the worst pain, average pain, and present pain scores were statistically significant compared with before treatment, with t = 2.26, 3.24, 2.02 and p = 0.029, 0.002, 0.049), and then rebounded. There was no statistically significant difference in the worst pain, lightest pain, average pain, and present pain scores between pre- and post-treatment at 12 weeks after treatment (t = 1.95, 0.40, 0.68, 0.93; p = 0.057, 0.693, 0.502, 0.355).
Comparison between groups
After 3 days and 4 weeks of treatment, the worst pain scores of both groups decreased to varying degrees, and the difference between the two groups was statistically significant (t = 2.04, 2.14; p = 0.047, 0.038). After 12 weeks of treatment, there was a statistically significant difference in the worst pain, average pain, and present pain scores between the two groups (t = 2.04-3.41; p = 0.001-0.047) (Table 3, Figure 3).
Table 3
Pain-related variables and scores in different therapy strategies
Bone pain affected survival areas
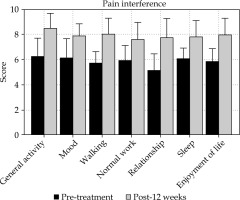
After 12 weeks of treatment, bone pain affected seven aspects of patient survival, including daily activities, emotions, walking ability, daily work, social activities, sleep quality, and interest in life. The interference scores are shown in Table 3. With the relief of bone pain, the degree of pain impact on both groups of patients decreased and enjoyment of life improved. Post-treatment, the scores of group A were higher than those of group B, and the difference was statistically significant (t = 2.04~3.16; p = 0.022~0.047) (Table 4, Figure 4).
Table 4
Pain interference-related variables in different therapy strategies
Analysis of influencing factors related to the relief of bone pain
In both univariate and multivariate analyses, the results showed that pre-operative worst pain score and Gleason grading were independently correlated factors, with relative risk (OR) values of 1.967 (p = 0.013, < 0.05) and 2.273 (p = 0.041, < 0.05), respectively (Table 5). According to the sub-group analysis of pre-operative worst pain score and Gleason grading, among the 14 cases with pre-operative worst pain score ≥ 7, the recurrence time of pain was 13.2 weeks. Among the 9 cases with pre-operative worst pain scores < 7, the recurrence time of pain was 14.1 weeks (χ2 = 7.22; p = 0.013, < 0.05). The pain recurrence time of 11 patients with Gleason score ≥ 7 and 12 patients with Gleason score < 7 were 12.8 and 14.5 weeks, respectively (χ2 = 3.26; p = 0.041, < 0.05). After further analysis, the pain recurrence time of 7 patients with the worst pain score and Gleason score ≥ 7 and5 patients with the worst pain score and Gleason score < 7 were 12.5 and 15.2 weeks, respectively (χ2 = 2.76; p = 0.045, < 0.05).
Tumor marker
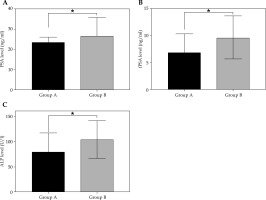
Prostate specific antigen, fPSA, and ALP were significantly lower in group A than in group B at 12 weeks post-operatively, and the difference was statistically significant (t = 4.38, 6.82, 4.97; p = 0.042, 0.012, 0.031) (Figure 5).
Fig. 5
A) Comparison of PSA, fPSA, and ALP values after treatment in groups A and B (x ±s). PSA was 19.34 ±6.83 ng/ml and 26.76 ±9.16 ng/ml in both groups, respectively, with statistically significant differences (t = 4.378, p = 0.042). B) fPSA was 6.83 ±3.51 ng/ml and 9.67 ±3.98 ng/ml, respectively, with statistically significant differences (t = 6.823, p = 0.012). C) ALP was 80.24 ±37.42 U/l and 104.38 ±37.53 U/l, respectively, with statistically significant differences (t = 4.970, p = 0.031)
Discussion
Previously, all patients enrolled in this study have received radiotherapy for painful bone metastases with poor analgesia, and required long-term dependence on strong opioids. Specific drugs, including morphine extended-release tablets (60-120 mg/day) or fentanyl transdermal patches (25-50 μg/h) were converted to MEDD by the morphine equivalent dose conversion table [9]. The process of prostate cancer bone metastasis is divided into four stages, i.e., colonization, dormancy, reactivation, and proliferation [10]. Reactivated tumor cells can enter the proliferation phase. In the proliferation phase, tumor cells can release a variety of cytokines to activate osteoblasts and osteoclasts. Therefore, targeted internal radiotherapy for tumor cells in the reactivation and proliferation phase will achieve good curative effect. In this paper, 125I internal radiotherapy was applied to treat moderate to worst pain in patients with prostate cancer bone metastases after failure of external irradiation. It was found that 125I implantation combined with 89SrCl2 provided more effective analgesic effect for patients with prostate cancer bone metastases after unsuccessful external irradiation and radiotherapy. The worst pain, average pain, and present pain scores in group A were significantly lower than those in group B at 12 weeks after treatment, and PSA, fPSA, and ALP in group A were significantly lower than those in group B at 12 weeks post-procedure (t = 4.38, 6.82, 4.97; p = 0.042, 0.012, 0.031). In a study, 42 patients with bone metastasis pain were treated with conventional dose fractionated radiotherapy and 43 patients were treated with radionuclide therapy [11]. The effective relief time of radiotherapy group (13.86 ±2.61 days) was significantly longer than that of radionuclide therapy group (9.53 ±2.72 days; p < 0.05), and the analgesic effect of radiotherapy group was relatively weaker than that of radionuclide therapy group. In this study, the worst pain in group A at 3 days, 4 weeks, 8 weeks, and 12 weeks after treatment was compared with that before treatment. According to the statistics, t = 2.01 and p = 0.0501 was close to p < 0.05 at 3 days after treatment, and there was statistical significance noted at 4 weeks, 8 weeks, and 12 weeks post-procedure (t = 7.11, 5.31, 6.14; p < 0.0001), indicating that the worst pain was significantly improved at all time periods after seed implantation, suggesting that the combined treatment for explosive pain has obvious advantages. This study also discussed the bone pain interference score among prostate cancer patients with pain due to bone metastases. After treatment, the scores of patients in group A were higher than before treatment, and higher than the scores of patients in group B (t = 2.04~7.34; all p < 0.05).
Radium-223 (223Ra) is approved for the treatment of patients with castration-resistant prostate cancer (CRPC), symptomatic bone metastases, and no known visceral metastases; it is indicated to improve survival in CRPC patients with bone metastases. While bone deposition radiopharmaceuticals for nuclide therapy have long been used to relieve pain in patients with bone metastases; the bone-seeking and favorable physical properties of alpha emitter 223Ra provided an additional survival advantage in the phase III trial ALSYMPCA. Whereas the combined mode of 125I and 89Sr has been preliminarily explored [12-14]. 125I seeds provide sustained low-dose radiation targeting localized high-risk foci (e.g., foci of risk for load-bearing bone and spinal cord compression) to prevent pathologic fractures; although 89Sr controls progression and pain in systemic multifocal lesions and reduces subsequent bone-related events (SREs). If 223Ra source is replaced, the combination of its alpha radiation with gamma/X-rays from 125I lacks support in the literature, and may increase the risk of radiation toxicity (e.g., myelosuppression superimposed). Most of the current studies investigating combination regimens for 223Ra focused on novel endocrine agents (e.g., abiraterone) or chemotherapy, but there are not sufficient data on safety and dosimetry of combining it with localized radiotherapy (e.g., 125I seed implantation). The 125I implantation treatment produces analgesia, and lesion and tumor marker reductions without causing radio-edema by continuously emitting low-dose γ-rays in close proximity, while continuously emitting pure β-rays in conjunction with 89SrCl2 systemic therapy. During the study period, in the pain level scores of group A at 12 weeks post-treatment, the worst pain, average pain, and present pain were still lower than that of the pre-treatment period (t = 6.14, 3.36, 2.86; p < 0.001, 0.002, 0.007), indicating that the group A treatment had a better effect on pain control and lasted for a long period of time. Whereas in group B, the scores firstly declined at 8 weeks post-treatment (the difference between the worst pain, average pain, and present pain scores compared with pre-treatment was statistically significant, with t = 2.26, 3.24, 2.02 and p = 0.029, 0.002, 0.049) and then rebounded. However, at 12 weeks after treatment, there was no statistically significant difference between the four levels of pain scores compared with pre-treatment (t = 1.95, 0.40, 0.68, 0.93; p = 0.057, 0.693, 0.502, 0.355). This result may be related to the long half-life of 59.6 days for 125I seeds and 50.5 days for 89SrCl2, and the fact that bone metastases can continuously receive γ-rays of 90-140 Gy and pure β-rays of 1.46 MeV. 89Sr emits pure β-rays with an energy of 1.4 GMeV and a half-life of 50.6 days. The biochemical properties of 89Sr are similar to those of calcium, which disappears from the bloodstream soon after intravenous injection and accumulates in osteoblastic tissue. Accumulation of 89Sr at the site of bone tumors reaches 1 smooth peak 10S after injection, while in the neighboring normal bone tissue, the accumulation of 89Sr reaches a peak at 1 day after injection and then decreases rapidly. The γ-rays of 125I seeds not only destroy DNA synthesis in the nucleus of tumor cells, inhibit mitosis, and induce apoptosis of tumor cells, but also make the tumor cells stay in the G2 M phase of the cell cycle, which is sensitive to rays, and then the tumor cells are destroyed by the maximum degree of radioactivity. Moreover, 125I internal radiation therapy can be used as a high-dose radiotherapy methods, and its radiation can be delivered in multiple sessions [15], with prolongation of irradiation time, cumulative dose, and efficacy of the tumor increase. In this study, the inclusion of vertebral bone metastases accounted for 51.28% (20/39). If the radiation dose is too high, it can easily damage the spinal cord; therefore, the 125I seeds show their own advantages with effective radius of 1.7 cm. By adjusting the position of radiation seeds, the overlapping radiation dose can effectively cover all tumor as well as sub-clinical regions within the normal tissues surrounding the tumor to achieve a high radiation within the tumor dose. The rapid attenuation of the radiation dose within the surrounding normal tissues without increasing radioactive damage to the adjacent important tissues, such as the spinal cord, will maximize the killing of tumor tissues and protect the surrounding normal tissues [16-18]. At the end of follow-up, some patients with bone metastases experienced pain recurrence, and we considered that it was mainly due to the attenuation of 125I radiation. Therefore, a second 125I seed implantation could be considered.
Radionuclide therapy is also used for bone metastases to reduce pain, kill tumor cells, prolong life, and improve quality of life. Radionuclide therapy using α- and β-emitting radionuclides is more targeted and effective than other local and systemic therapies. In recent years, α-emitting radiopharmaceuticals, e.g., radium 223 chloride (223Ra) and β-emitting radiopharmaceuticals, such as 89SrCl2, lutetium-177 (177Lu)-labeled ethylenediamine tetra-methylene phosphonate (EDTMP), and samarium-153 (153Sm)-labeled EDTMP, have been introduced into the clinic, particularly for the treatment of painful bone metastases. On the other hand, new radiopharmaceutical development studies are intensively being conducted, e.g., on actinium-225 labeled prostate-specific membrane antigen-617 (225Ac-PSMA). Moreover, many studies have demonstrated that the use of radiopharmaceuticals for the treatment of bone metastases improves the overall health of patients, reduces the risk of pain and pathologic fractures, and improves survival [19]. The application of 89SrCl2 for the treatment of bone tumors in this study has been researched and applied for more than 70 years; 89SrCl2 was clinically used in the United Kingdom and the United States in 1989 and 1993, respectively. The 89SrCl2 produced in China was approved by the State Drug Administration for clinical application in 2004 [20, 21]. 89SrCl2 emits β-rays, which focus on irradiating diseased tissues, inhibiting and killing tumor cells, exerting the effects of relieving bone pain, and inhibiting the growth of bone metastases [22]. It is currently believed that the possible mechanisms of pain relief include: 1. 89SrCl2 treatment shrinks the tumor and reduces the pressure on the affected periosteum and bone marrow cavity; 2. The radiation bio-effect interferes with the process of depolarization of nerve end-points, which affects the conduction of pain signals; and 3. The radiation bio-effect inhibits the production of inflammatory pain mediators, such as bradykinin and prostaglandins.
According to the unifactorial and multifactorial analyses of factors affecting bone pain relief, the pre-operative worst pain score and Gleason grading performance were independent correlates, and the relative risk (OR) was 1.967 (p = 0.013, < 0.05) and 2.273 (p = 0.041, < 0.05), respectively. Upon further analysis, the pain recurrence time was 13.2 weeks in cases with pre-operative worst pain score ≥ 7, and 14.1 weeks in 9 cases with pre-operative worst pain score < 7. In the sub-group of Gleason’s classification, the time of pain recurrence was relatively shorter in the patients with highest preoperative pain and Gleason’s risk classification score. The higher the Gleason risk score, the shorter the time to pain recurrence. This study provides a good basis for 125I implantation combined with 89SrCl2 for the treatment of prostate cancer bone metastasis patients, for whom external irradiation therapy was unsuccessful. However, in the course of treatment and follow-up, it was found that the main bone metastatic lesions included in this study were those with a diameter of less than 5 cm. There is a question whether it is feasible for giant-type lesions, lesions with irregular mass boundaries, and those close to the spinal cord, important blood vessels and organs, and other dangerous areas. As for bone metastatic lesions of other tumors, osteolytic lesions may predominate. Also, on the basis of previous treatment combined with bisphosphonates, will it increase the occurrence of adverse reactions and affect the tolerance of the patient? How to deal with it? In order to address the above questions, further research must be conducted among bone metastases patients with solid tumors, which affect the spatial distribution of radioactive seeds and make the dose distribution uneven for systematic analysis.
In the current study, in the treatment of CT-guided radioactive 125I implantation combined with 89Sr injection, patient pain relief was prolonged up to 12 weeks post-treatment, and was relatively more effective in targeting breakthrough pain, thus reducing psychological and physical pain associated with adverse effects. Additionally, it improved the survival domains affecting the seven dimensions of bone pain, including activities of daily living, mood, ability to walk, daily routine, social activities, quality of sleep, and interest in life, therefore improving the overall quality of life.
In conclusion, CT-guided 125I implantation combined with 89SrCl2 treatment is a feasible and effective therapy for the relief of pain caused by prostate cancer bone metastases after failure of external radiation radiotherapy.