Purpose
External beam radiation with brachytherapy (ICBT) has been the standard of care in patients with carcinoma cervix stage IB2-IVA. There are no substitutes to brachytherapy in the management of cervix carcinoma [1-5]. Brachytherapy follows the inverse square law, i.e., small increase in the distance from radiation source will cause a significant reduction of dose due to a high-dose gradient. This helps in reduction of doses to organs at risk (OARs), while simultaneously achieving high-dose to target volume. While low-dose-rate (LDR) brachytherapy was the standard of care previously, modern brachytherapy is almost entirely carried out with high-dose-rate (HDR) brachytherapy globally [5-7].
Considering identical dose distributions, HDR brachytherapy is radiobiologically inferior to LDR brachy-therapy. However, modern automatic afterloader stepping source-based HDR brachytherapy present distinct advantages, including the ability to treat a large number of patients on an outpatient basis, conformal dose distribution due to the possibility of optimization, shorter treatment time, and thus better patient compliance, etc. Modern HDR brachytherapy has been shown to be equal to LDR brachytherapy in terms of toxicity and local control [8-12].
Two most common late toxicities after therapeutic irradiation of cervix carcinoma are radiation proctitis and cystitis. Moderate to severe complications from HDR brachytherapy have been reported, with an incidence ranging from 5% to 30% [3, 8, 13-21]. Radiation-induced proctopathy most often manifests due to injury of anterior rectal wall, resulting in rectal bleeding, fibrosis, chronic rectal ulcers, and fistula formation [15-17]. Doses received by the bladder and rectum correlate with the incidence of such late toxicities [17, 22, 23]. Reduction in doses to these OARs is conventionally achieved with gauze packing of the vaginal cavity during brachytherapy procedure. Various methods to distance these organs away from high-dose regions have been attempted. These include simple techniques, such as vaginal gauze packing, speculum-based vaginal packing (SBVP), modified bladder and rectal spacer balloons, commercially available rectal retractors, and more invasive techniques, including instillation of hydro-colloid material posterior to the cervix (in the Douglas pouch) [24-33]. Rectal retractor has been shown to reduce the rectal dose significantly [33-35]. Previous studies using tandem and ovoid applicators have evaluated the use of a balloon from a generic commercial Foley’s catheter as an intra-vaginal balloon in addition to gauze packing. They demonstrated that significant reduction in the dose to the rectum and bladder was achieved without affecting the tumor dose coverage [26-30]. In this study, we dosimetrically compared the rectal and bladder doses between intra-vaginal balloon plus gauze packing and gauze packing using tandem and ring applicators.
Material and methods
Eligibility
Patients with uterine cervical cancer from stage IB to IIIB, who have completed EBRT and received at least 45 Gy, and were found suitable for intracavitary brachytherapy using tandem and ring applicators, were included in the study. Patients with poor performance status (ECOG PS > 2), with history of pelvic radiation prior to the present course of treatment or pelvic surgery, and patients aged less than 18 years were excluded from the study.
Design
Forty-five patients were randomized using SNOSE method into group A or B after obtaining informed consent. Consecutive sampling was done to include the patients. Our institution practice for HDR brachytherapy delivery after external beam radiotherapy was 8.5 Gy in 3 fractions when the study was being conducted. Each patient received three sessions of HDR brachytherapy at 8.5 Gy per session. Data collected from the first two sessions were included in the analysis, while the third fractions were delivered according to standard institutional practice. The current study was approved by the Institute Ethics Committee, JIPMER, Puducherry (JIP/IEC/2016/1052) and this trial was registered with CTRI/2018/04/013460.
Objectives
The primary objective was to compare D0.1cc, D0.5cc, D1cc, and D2cc of the rectum and bladder estimated from brachytherapy plan between the two different vaginal packing techniques. The secondary objective was to compare the same dose measures in treatment plans generated from pre- versus post-brachytherapy computed tomography (CT) imaging for each brachytherapy session.
Patient assessment for brachytherapy
Per vaginal and per rectal examination were performed to assess the disease and vaginal length, and details were recorded. Vaginal length was measured from the anterior fornix to the introitus with per vaginal examination, and correlated with a ruler. Patients were sounded with uterine sound with trans-abdominal ultrasound guidance to estimate the appropriate applicator angle and length.
Randomization
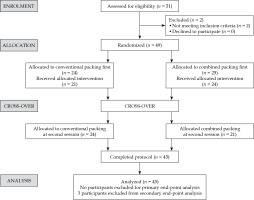
At enrolment, patients found suitable for tandem and ring-based brachytherapy were randomized into group A and group B with block randomization using serially numbered opaque sealed envelope (SNOSE), and assigned a weekly schedule for brachytherapy procedures. Group A underwent the first session of brachytherapy with combined packing, and the second session with vaginal gauze packing alone. Group B underwent the first session with vaginal gauze packing alone, and the second session with combined packing, as illustrated in consort diagram in Figure 1.
Brachytherapy
For all patients, the procedure was done under aseptic precaution in the brachytherapy minor operation theatre, with the patient in lithotomy position, and intra-muscular analgesics and sedatives were administered prior to the start of procedure.
Combined vaginal packing
Ring and tandem applicator set of appropriate length and angle from Varian Inc. was selected after verifying morphology and uterine length by clinical examination, uterine sound assessment, and trans-abdominal ultrasound. A standard ring size of 32 mm was applied for all the patients.
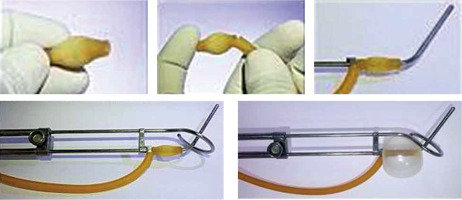
The tip of a 20 Fr Foley’s catheter was cut-off distally to the balloon. The main lumen (for urinary drainage) of the Foley’s catheter was opened by a small nick proximally to the balloon to allow the tandem to be threaded into this lumen. The balloon was slid down the tandem and positioned to occupy the stem of tandem from the flange till the lock of tandem (as shown in Figure 2).
The tandem was inserted into the uterus, and its’ position in the uterus was verified by abdominal ultrasonography. Subsequently, the ring was secured in place and locked onto the tandem. The ring cap was not added to the applicator in clinical situation where vaginal cavity was assessed to be narrow or highly restrictive due to poor elasticity, fearing vaginal tear (although we do not recommend this as the routine way of securing the applicator). The same applicator arrangement was repeated in the second session of brachytherapy in both the groups. The intra-vaginal balloon was partially inflated with 15 cc of diluted iohexol. Vaginal cavity was packed with acriflavine-soaked gauze roll to separate the anterior vaginal wall from the stem of the ring and the posterior vaginal wall from the stem of the tandem as well as laterally to completely secure the applicators in right position. Vaginal packing was continued in this manner till the introitus. Finally, the balloon of Foley’s catheter attached to the tandem was inflated with additional 15 cc of diluted iohexol. Proper expansion of the Foley’s balloon was ensured by simultaneous per rectal examination. The patient was assessed for excessive pain during inflation of the balloon. A rectal tube was inserted after lubrication, and the applicator position was secured using T bandage. Finally, the patient position was changed from lithotomy to neutral (supine) position.
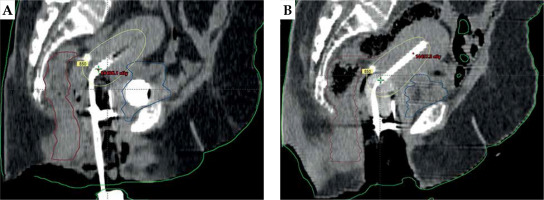
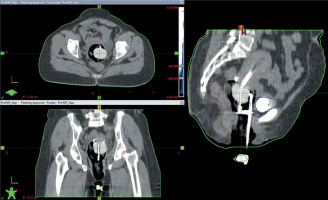
Following applicator insertion, the patient was shifted to CT simulator. CT simulation was done using Siemens Somatom Spirit dual slice CT simulator. CT scan extent was from L4-L5 interspace superiorly to at least 3 cm below the introitus, with slice thickness of 3 mm. CT scans were assessed for proper application, emptying the bladder, and position of the balloon in relation to the tandem. If the image revealed improper position of the applicator or the balloon, relevant steps were repeated. Finalized CT scans were transferred to Eclipse treatment planning system (TPS) version 10.0.28 (Varian Medical Systems, Palo alto, CA, USA). In TPS, dose calculation was accomplished using AAPM TG43 formalism. A medical physicist reconstructed the applicators, activated dwell positions, and the plan was normalized to point A, as defined by the American Brachytherapy Society (ABS). Bladder and rectal points were placed according to ICRU 89. The prescribed dose to point A was 8.5 Gy per session. Generated brachytherapy plans were evaluated by an experienced radiation oncologist. Dwell positions and times were manually optimized to cover the disease in the cervix and vagina, and doses to the bladder and rectum were minimized. A comparison of sagittal CT images of gauze packing alone (Figure 3A) vs. combined packing (Figure 3B) is shown in Figure 3. Multiplanar CT images of combined packing are shown in Figure 4. Patients were treated with optimized plans, and HDR brachytherapy was delivered with 192Ir-based afterloader, GammaMed Plus iX® (Varian Medical Systems, Palo alto, CA, USA).
After delivery of brachytherapy, the patient was immediately shifted to the CT room for a post-treatment CT scan using the same CT simulator mentioned previously. Post-treatment CT scans were transferred to the same TPS, and after applicator reconstruction, dwell positions and dwell times from the treated plan were replicated in the post-treatment plan to ascertain dosimetric variation of OARs. After completion of the CT scan, the applicators were removed, the bladder was de-catheterized, and patients were observed until they recovered completely before being discharged. Procedural notes and procedure-related complications during application and removal were assessed.
For reporting the bladder and rectal doses, the bladder and rectum were later contoured using RTOG pelvis contouring guidelines and ICRU point dose, and volumetric doses of D0.1cc, D0.5cc, D1cc, and D2cc for both the rectum and bladder were collected in terms of absolute dose from both pre- and post-treatment CT scans.
Conventional vaginal gauze packing
After uterine sounding, the tandem was placed into the uterine cavity and its’ position was verified by ultrasonography. Then, the ring was secured in place and locked onto the tandem. The vaginal cavity was then packed with acriflavine-soaked gauze roll first anteriorly and posteriorly, and then laterally till the introitus. A rectal tube was inserted after lubrication. Applicator position was secured using T bandage. The same procedure of CT simulation as well as planning and execution of the treatment was followed according to the above-mentioned technique.
Statistical analysis
Assuming alpha error of 5%, power of 80% with mean rectal dose, and a standard deviation (SD) as 71.2 ±11.5 and 66.9 ±9 in standard packing technique vs. combined packing technique, respectively, it was calculated to study 36 pairs of plans from 36 patients. Considering a non-compliance rate of 20%, forty-four patients were planned for recruitment in the study. The sample size was calculated using PS: Power and sample size calculation version 3.0, Vanderbilt. P-value of < 0.05 was considered statistically significant.
Results
Patients’ characteristics
Fifty-one patients were screened, two were excluded prior to randomization, and four patients defaulted after randomization. A total of 45 patients and 90 self-matched CT data sets were included in the analysis. Patient and disease characteristics are summarized in Table 1. All 45 patients had one session of HDR brachytherapy with combined packing, and one session with gauze packing due to cross-over design. Based on the gynecological assessment of our study patients, we found all our patients to have reduced elasticity of the upper vagina and/or a narrow vaginal cavity following pelvic irradiation. Therefore, the ring cap was not used in all of the patients to facilitate appropriate geometric application and to avoid possible vaginal tear. The applicator arrangement in the first session was repeated in the second session of brachytherapy in both the groups.
Table 1
Baseline characteristics of the patients
Dosimetric comparison
Comparative results of OARs doses between combined and conventional packing techniques are summarized in Table 2. The urinary bladder was contoured as the outer wall of the bladder. Dose volume parameters for urinary bladder D0.5cc, D1cc, and D2cc and ICRU bladder points were expressed as mean and SD, while D0.1cc was expressed as median and range. In the combined packing group, the median D0.1cc of the bladder was 11.20 Gy (range, 4.89-24.42 Gy). In the conventional packing group, the median D0.1cc of the bladder was 10.76 Gy (range, 4.41-34.79 Gy). D0.1cc was higher in the combined packing group compared with conventional packing (11.20 Gy vs. 10.76 Gy), which was not statistically significant (p = 0.18). Even other parameters, such as D0.5cc, D1cc, and D2cc as well as ICRU bladder point doses were higher in the combined group, but were statistically not significant.
Table 2
Dosimetric results of organs at risk (OARs) doses between two packing techniques
The rectum was also contoured as the outer wall of the rectum. D0.1cc, D0.5cc, D1cc, D2cc, and dose received by ICRU recto-vaginal (RV) points were expressed as mean and SD. D0.1cc, D0.5cc, D1cc, and D2cc were less in combined packing compared with conventional packing, and the difference was statistically significant. ICRU RV point dose was less in combined packing (5.14 Gy vs. 5.28 Gy), but it was not statistically significant (p = 0.09).
Intrafraction variation
In the combined packing technique, we observed no intrafraction variation in the rectal doses, while some of the bladder dose values had changed. Changes in the rectal and bladder doses between brachytherapy treatment plans generated using CT imaging taken before and after brachytherapy session are presented in Tables 3 and 4 for both packing techniques.
Table 3
Organs at risk (OARs) doses of pre- and post-treatment plans in combined packing and their comparison
Table 4
Organs at risk (OARs) doses of pre- and post-treatment plans in conventional packing and their comparison
Complications
Converting the standard Foley’s catheter by appropriately incising the tube at two locations was relatively easy. However, in case where the proximal incision was made too deep, or if the distal incision cut through the balloon’s inflation channel, the balloon could not be inflated, rendering that catheter unusable. One patient, who was supposed to be in the inflated balloon arm was noted to have a deflated balloon while removing the applicator, possibly due to balloon’s integrity getting damaged during gauze packing, as the balloon was partially inflated prior to gauze packing. Three patients (6.6%) had complications, including uncomplicated uterine perforation, vaginal mucosal denudation, and urethral inflammation. No patient had a vaginal or rectal tear. We did not observe any excess pain in patients undergoing combined packing. However, patients who reported pain scores were not assessed objectively, and hence it would be difficult to evaluate patients’ comfort using different packing techniques.
Discussion
Dose to OARs in HDR brachytherapy is of significant concern since there is negligible intrafraction repair of normal tissues. In clinical practice, there are various techniques to decrease doses to OARs. The present standard of care is packing vagina with a roll of cotton gauze to push the vaginal walls and thus displace the adjacent OARs. In our study, the Foley’s balloon was combined with conventional gauze packing, as the added gauze helped to stretch the vaginal mucosa thoroughly and fill any gap between the balloon and the vaginal wall. Modified Foley’s catheter, like the one used in our study, was already studied using tandem and ring applicators, but novelty of our study is to combine it with gauze packing, while the other studies compared balloon alone versus gauze packing. Here, we intended to study the additive effect of balloon plus gauze compared with gauze packing.
Eng et al. from the University of Texas, investigated 22 patients, in whom two Foley’s balloons, anteriorly and posteriorly to tandem along with vaginal gauze packing were placed [27]. They analyzed conventional radiography-based treatment plans done with and without inflation of balloons, and showed a mean reduction in ICRU bladder and rectal point doses of 16% and 17.6%, respectively. The authors showed that the inflated Foley’s balloon provided an additional advantage of case-by-case customizability, with a low-cost implementation. CT-based treatment plans using the same study design in 38 patients showed 7.2% of reduction in the bladder dose (D0.1cc, D1cc, and D2cc) and 9.3% reduction in the rectal dose (D0.1cc, D1cc, and D2cc) with inflation of the Foley’s balloon combined with conventional vaginal packing [28].
In the current study, reductions of 16.6% in D0.1cc, 12.8% in D0.5cc, 11.77% in D1cc, and 11.3% in D2cc of the rectum were observed. The anterior expansion of the balloon was limited by the stem of the ring. Placing vaginal gauze packs between the stem of the ring and the anterior vaginal wall was used to compensate for the balloon’s limited reach. Despite this, we could not find any difference in the bladder dose between the groups.
Rai et al. in a randomized controlled trial with cross-over design used a commercially available bladder-rectum spacer balloon, and compared with vaginal gauze packing. In this study, rectal ICRU point of D0.1cc, D1cc, D2cc, D5cc, and D10cc to be 5.05 Gy, 7.32 Gy, 5.58 Gy, 5.06 Gy, 4.21 Gy, and 3.50 Gy, respectively, in gauze packing when compared with 4.82 Gy, 6.33 Gy, 5.05 Gy, 4.63 Gy, 4.07 Gy, and 3.41 Gy with BRSB packing, which was statistically significant, but there were no significant differences in the bladder doses or D5cc and D10cc of the rectum with BRSB [29]. BRSB was applied with minimal gauze packing distally to the balloon, to immobilize the applicator and prevent its’ slippage. Our packing technique gives the radiation oncologist the freedom to pack in the traditional manner, and later increase the volume of intra-vaginal packing by final inflation of the balloon after completion of gauze packing. The gauze packing remains both, anteriorly and posteriorly to the inflated balloon and not just distally. Moreover, tandem and ovoid applicators can be used with BRSB, but tandem and ring cannot be applied with BRSB as the inflation of the balloon displaces the ring.
Few studies had shown reduction in rectal dose with rectal retractors over conventional packing [31, 33]. Though commercially available rectal retractor can be an effective way to reduce the rectal dose, the use of rectal retractor is highly restrictive in patients with a narrow vagina. In our study, we used an easily available Foley’s catheter suitably modified as a balloon to enable its’ inflation circumferentially around the stem of the tandem, while combining with gauze packing to provide maximal distancing of the rectum and bladder, similar to Foley’s tandem used in a Kong et al. study [35].
In the present study, the median rectal dose D2cc and sigmoid D2cc was found to be lesser with rectal retractor (1.31 Gy and 1.27 Gy) than inflated Foley’s’ balloon (1.99 Gy and 1.81 Gy) and gauze packing (2.18 Gy and 1.80 Gy), with a point A prescription dose of 26 Gy (6.5 Gy × 4 fractions).
We believe that this is the first randomized controlled trial using tandem and ring comparing combined Foley’s catheter balloon and gauze with vaginal gauze packing alone. We were able to reduce the bias involved in patients’ selection by appropriate randomization. Since we used a cross-over design, each patient underwent investigational procedure as well as conventional procedure, and therefore acted as their own internal control. This eliminated confounding due to anatomical variation. Ring caps were not used in the study. We agree that not using ring caps would lead to high vaginal doses, which is also not a favored option. As an institutional practice, we avoid placing ring caps only in clinical situations with narrow vaginas following pelvic EBRT. In the past, we observed that such patients developed upper vaginal fibrosis and/or stenosis without any fistula formation. CT-based treatment plans were used for dosimetric analysis and we reported dose-volume parameters that correlated more with clinical toxicities. All the procedures were done by the investigators, thereby ensuring consistency in the techniques used. While patients were blinded to the packing technique, blinding the physician or physicist was not feasible due to the obvious appearance of additional Foley’s balloon in the images. We attempted to ensure consistency in the combined packing arm by filling the Foley’s balloon with a consistent 30 cc of fluid. In one patient, we observed that only 25 cc could be inflated, after which she developed pain and the rectum distension was judged to be adequate. However, further analysis may be necessary to explore whether patient-specific customized filling and distension of the vaginal packing results in even better reduction in the rectal dose.
Rai et al. had observed a complication rate of 2.3%, which included minor vaginal tear, while there were no major complications observed. We had a higher complication rate of 6.6%, but all of them were minor and self-limiting.
The intrafraction dose variation was studied by comparing the doses calculated from CT imaging done before and after the brachytherapy treatment. This suggests the stability of the application until the completion of treatment. We found no intrafraction variation in the rectal doses in both the packing techniques. However, the bladder doses changed in the combined packing technique. The gauze added between the stem of the ring and the anterior vaginal wall was the only way of spacing the bladder away from the tandem. Nevertheless, since the change in the bladder dose was observed only in the combined packing, a possible explanation could be that the presence of a distended balloon posteriorly resulted in unstable anterior packing. The clinical impact in terms of late effects could not be evaluated due to the cross-over design of the study. Also, cost-effective analysis and time required for the packing techniques were not analyzed.
We can therefore conclude from all the related studies that the utility of balloon in various forms of vaginal packing resulted in reducing the rectal and bladder doses. Rectal retractors have also shown to decrease rectal dose significantly. Our combined packing technique resulted in reduced rectal dose without affecting the bladder dose.
Conclusions
This dosimetric study showed that combined packing reduced the rectal dose over gauze packing alone, with no change in the bladder dose. The applicator was found to be stable in the context of rectal dose distribution. A parallel study design will help to evaluate the clinical impact of this combined packing technique.