Purpose
Breast cancer is the second most common type of cancer in females, causing 11.6% of all cancer cases and 6.9% of cancer-related deaths worldwide, according to global cancer statistics (GLOBOCAN) 2022 data analysis [1]. Currently, adjuvant radiation therapy (RT) to whole breast is the standard of care for early-stage breast cancer patients after breast-conserving surgery (BCS) [2-5]. Adjuvant RT reduces the likelihood of local recurrence (LR) by 2/3, and increases 20 years of absolute survival benefit by 2-4% [6]. Approximately 3% to 15% of ipsilateral breast tumor recurrences (IBTRs) occur near the tumor cavity, requiring additional radiation to maintain local control [7-9]. Numerous randomized controlled trials (RCTs) have shown how crucial tumor bed boosts are for improving local control (LC) and achieving cosmetically acceptable results. Comparing patients treated with a boost with those without a boost, the 5-year local recurrence rate (LR-rate) decreased from 7.3-13.3% to 3.6-6.3% [10, 11].
The GEC-ESTRO recommendations guided the creation of three categories for tumor bed boost. Patients in the intermediate-risk and high-risk groups achieved better local control with a boost. This intermediate group included patients under 40 years of age without any major risk factors, those aged > 40 years and < 50 years regardless of any risk factors, and subjects of over 50 years of age, who did have any risk factors, such as multicentric or multifocal tumors, tumor size > 3 cm, lymph node invasion, extensive intra-ductal component (EIC), lympho-vascular invasion (LVI), close margins, triple-negative breast cancer (TNBC) phenotype, or post-neoadjuvant chemotherapy (NACT) in case of a residual tumor. A 10 to 16 Gy EQD2 boost is considered optional for these individuals. High-risk patients, especially those under 40 with risk factors, require dose escalation above 16 Gy EQD2 [12].
A tumor bed boost is delivered using external beam radiotherapy (EBRT) and interstitial brachytherapy (BT). Surface tumors within 4 cm of the skin, receive electron boosts, while deeper tumors require higher electron energies and increased skin dose. For large or deep-seated tumors, both multicatheter interstitial brachytherapy (MIBT) and intensity-modulated radiotherapy (IMRT)/volumetric modulated arc therapy (VMAT) perform efficiently. Compared with 3D-CRT, IMRT offers a more homogeneous dose distribution, reducing skin reactions and doses to organs at risk (OARs). However, its disadvantage include a potential for increased secondary cancer risks due to low OARs doses. MIBT, which may offer better long-term cosmetic outcomes and reduced skin toxicity, involves specialized brachytherapy expertise. However, several trials have shown that high boost doses can worsen cosmetic outcomes.
Many studies have examined the dosimetry for 3D-CRT, IMRT, VMAT, simultaneous integrated boost (SIB), and MIBT boosts as well as toxicity and cosmesis associated with hypo-fractionated RT followed by HDR-BT. This study was the first to comprehensively analyze the dosimetry and clinical outcomes of IMRT and MIBT photon boost after whole breast hypofractionated radiation in post-BCS patients. The study compared several parameters, including dosimetry, acute and late toxicity, cosmesis, loco-regional recurrence (LRR), progression-free survival (PFS), and overall survival (OS) between two patient groups (i.e., IMRT and MIBT).
Material and methods
Study design
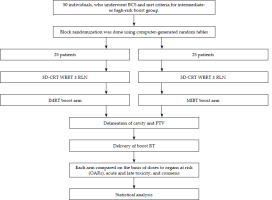
Figure 1 summarizes the study design. This mono-institutional, randomized, prospective control study (RCT) was conducted in the Department of Radiotherapy and Clinical Oncology at PGIMER, Chandigarh, India. The study’s preliminary work started in September 2018 under the guidance of Prof. Rakesh Kapoor, and the official ethics committee approved its protocol on April 4, 2019 (approval number: 10604). In total, fifty (n = 50) subjects, who had undergone a lumpectomy were enrolled in this study between September 2018 and Jan 2020. Patients were randomized using computer-generated random numbers. Before starting whole breast radiotherapy (WBRT), participants were randomly assigned into two treatment groups, with doctors blinded for group allocation. Female patients aged 30-60 years, with a primary breast cancer, Karnofsky performance status (KPS) score of more than 70, hormone receptor-positive or negative (ER/PR/HER2/neu/Ki67), after chemotherapy or not, willing to participate, and who provided written informed consent for participation, were included. Subjects with poor general health, distant metastases, pregnancy or lactation, and a previous radiation therapy to the breast or chest wall were excluded.
3D-CRT simulation and planning
A planning CT scan, with a 3 mm slice thickness on each patient, placing markers along the breast borders, and inserting markers over surgery scars, was performed. Patient was placed in a supine position, and extended CT from the mandible to the lower border of the liver was attained. A breast board was applied to abduct the arm 90 degrees and rotate the supraclavicular fossa (SCF) to the opposite side. RTOG contouring atlas guidelines [13] were considered for delineating WBRT, utilizing Eclipse treatment planning system (TPS), version 11.3 (Varian ARIA external beam planning software) for precise planning and contouring. Supplementary Figure 1A-E illustrates the target delineation for 3D-CRT WBRT plus regional lymph-nodal radiation (RLN). Gross tumor volume (GTV) included a lumpectomy cavity contoured precisely on each CT slice. The lumpectomy cavity was characterized based on pre-operative reports from mammography and ultrasound, surgical descriptions and clips, and post-operative seroma cavities or fibrosis in CT scan. Clinical target volume (CTV-breast) encompassed the entire breast, including GTV. Radiopaque wires defined the field borders to exclude the pectoralis muscles, chest wall, ribs, and a 5 mm edge from the skin surface. Clinical target volume for nodal regions (CTV-nodal) included axillary, supraclavicular, and internal mammary lymph nodes as per clinical indications. Next, CTV-total was established using a Boolean combination of CTV-breast and CTV-nodal. Planning target volume (PTV) was created by applying a uniform 0.5 cm expansion around CTV-total to account for setup variations and patient movement. Final PTV (PTV-final) was adjusted by excluding a 5 mm margin from the skin surface to optimize sparing of surrounding healthy tissues.
Supplementary Figure 2A-D demonstrates the 3D-CRT planning for WBRT plus RLN. Patients were treated with a single isocenter technique and 6 MV photons, using two tangential beams on the breast, and either mixed or 6 MV fields on the area above the clavicle. Direct anterior field went over the supraclavicular and level III areas. In contrast, the two conformal tangential fields covered the whole breast and levels I and II in the axillary region. Prescription depth varied for the supraclavicular field based on patient’s thickness. The tangential lung field was adjusted to minimize central lung dose (CLD) and prevent damage to the contralateral breast. A dose of 40 Gy in 16 fractions (2.5 Gy per fraction) was delivered to PTV-final, five times a week. Treatment plans were evaluated, both qualitatively and quantitatively, for coverage of OARs doses and DVH parameters. Following WBRT, patients received either an IMRT or an MIBT boost.
IMRT boost tumor bed delineation and planning
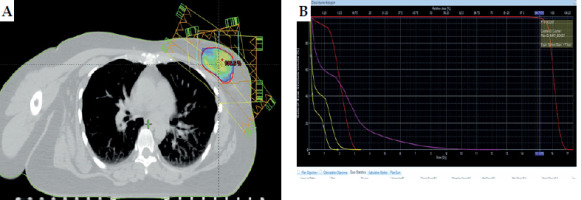
Figure 2A and B shows the IMRT boost planning. The same GTV from 3D-CRT planning CT was used as GTV-boost for the IMRT boost planning process. To create a CTV-boost, the GTV-boost was uniformly expanded by 1-1.5 cm in all directions while limiting it to 5 mm within the exterior contour, to minimize skin toxicity and avoid the pectoralis major muscles. PTV-boost was derived from the CTV-boost, incorporating an additional 0.5 cm expansion to account for setup and motion errors. The institutional protocol specifies a minimum safety margin of 1.5-2 cm from the tumor cavity. Planning and using an IMRT field-in-field technique (FIF) ensured the coverage of PTV-boost and adhering to the guidelines mentioned in the ICRU Report 62 for dose uniformity. The prescribed dose was 16 Gy in 8 fractions (2 Gy per fraction), delivered five times a week.
MIBT boosts tumor bed delineation and planning
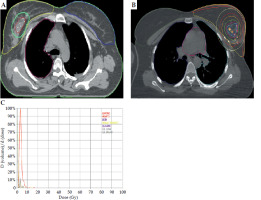
Supplementary Figure 3A-E illustrates the MIBT catheter insertion process. Key information for catheter insertion included clinical data, intra-operative findings, surgical clip locations, and pre-operative mammography images. WBRT planning CT scan recorded measurements of the tumor cavity, skin distance, and clip locations in axial, coronal, and sagittal planes. Ultrasound-guided (USG) catheter placement was employed to confirm that the needles were at least 1 cm from the skin, correctly positioned relative to the tumor cavity. The needles were inserted using blind or template-guided methods, replaced with plastic catheters, and secured using a micro plaster. After shifting the patient from operating theater (OT), planning CT was done for the patient, and the cavity was delineated, including the lumpectomy cavity, seroma, surgical clips, fibrosis, and adjacent OARs. The cavity was expanded uniformly by 1.5 cm in all directions to create PTV-boost (i.e., CTV-boost), but limiting it to 5 mm within the exterior contour to minimize skin toxicity. Institutional protocol was followed, giving a minimum safety margin of 1.5-2 cm from the tumor cavity [14]. PTV-boost was prescribed at 15 Gy in 5 fractions over 3 days, and delivered with two fractions per day. Copper wires were applied to reconstruct the catheters on the planning CT, source dwell positions were determined based on the Paris system, and geometrical and graphical optimization methods were used for dose planning. Planning goals were based on the GEC-ESTRO guidelines, which included limits on V100%, dose non-uniformity ratio, and maximum doses that could reach the skin, ribs, heart, and ipsilateral lung [15]. Treatments were either delivered with HDR Plus 3.0.6.0 (Eckert & Ziegler BEBIG GmbH) for cobalt-60, or Oncentra TPS version 4.3.0.410 (Nucletron, Elekta) for iridium-192 source. Figure 3A-C demonstrates the MIBT boost planning.
EQD2 calculation
In the current study, the α/β ratio was kept constant at 3 for the breast [16]. A medical physicist calculated EQD2 dose for WBRT plus boost in both groups, ensuring that the EQD2 dose was nearly equal due to different fractionation schedules. The cumulative EQD2 for acute tissues was 57.7 Gy and 57.9 Gy in WBRT plus IMRT and WBRT plus MIBT arms, while the cumulative EQD2 for late tissues was 59.9 Gy and 61.9 Gy in both arms, respectively. This was to avoid potential dose-related bias in evaluating cosmesis and toxicity during long-term follow-up.
Toxicity and cosmesis assessment
Both acute and late toxicity were assessed using the RTOG toxicity criteria [17]. Acute side effects were considered in patients, who showed toxicity within the first three months of radiation, and late toxicity was defined as toxicity effects occurring after six months post-treatment. To evaluate toxicity and cosmesis long-term, follow-up visits for each patient in the study were scheduled at 6 months, 1 year, 2 years, and 5 years post-treatment. A total of 10 patients within 6 months in June 2019, 35 patients within 1 year in February 2020, 48 patients within 2 years in March 2022, and 47 patients within 5 years in April 2024 were evaluated with side effects. The fluctuation in patient numbers at each time point was attributable to missed visits, deaths, and unavailability for follow-up visits.
Cosmesis was estimated using both patient self-assessment and physician assessment. In physician-reported cosmetic outcomes, Harvard criteria [18] were applied, classifying outcomes as excellent (4), good (3), fair (2), or poor (1), while patient-reported cosmetic outcomes were assessed based on the score provided by patient. One physician evaluated all cosmetic outcomes, whereas OS was defined as the duration from completion of radiation therapy to death from any cause or last follow-up. In contrast, PFS was specified as the time from completion of radiotherapy to disease recurrence or progression. Loco-regional recurrence (LRR) was considered as a rate at which the disease recured in the exact location or near the original site.
Statistical analysis
An independent t-test to compare the dosimetry results was employed, and acute and late toxicity as well as cosmesis were assessed using a likelihood ratio. Fisher exact test evaluated LRR, while Kaplan-Meier and log-rank tests estimated OS and PFS. Continuous data were reported as mean and standard deviation, assuming a normal distribution. Statistical significance was determined with a p-value threshold of < 0.05, with the null hypothesis stating no difference between the study arms. Data analysis was conducted with IBM SPSS Statistics, version 29.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Table 1 summarizes the primary patient characteristics, with additional details provided by Supplementary Table 1A. This study randomly assigned 25 patients to the IMRT and MIBT treatment groups (in total, 50 patients), with a median follow-up of 59.4 months. The mean ages were 45 years for the IMRT arm and 40 years for the MIBT arm. Tumors were predominantly located in the upper outer quadrant (UOQ), with most patients having early-stage breast cancer (cT1-T2, N0-1), except for one patient with cT4 stage; majority had infiltrating ductal carcinoma (IDC). The median tumor sizes were 2.9 cm in the IMRT group and 3.6 cm in the MIBT group. Positive LVI was observed in 6 of the 25 IMRT patients and 12 of the 25 MIBT patients. Perineural invasion (PNI) was present in 3 IMRT patients, but absent in MIBT patients. DCIS was found in 14 of the 25 IMRT and 9 of the 25 MIBT patients. ER positivity was observed in 14/25 IMRT and 7/25 MIBT patients, while 4/25 IMRT and 1/25 MIBT patients showed HER2 positivity. NACT was administered to 11/25 IMRT and 8/25 MIBT patients, and adjuvant chemotherapy to 11/25 IMRT and 16/25 MIBT patients. All HER2-positive patients received trastuzumab for one year. Risk classification for the breast boost showed 32% of high-risk patients in the IMRT arm and 56% in the MIBT arm. The mean breast volume was 968 cc and 884 cc, and the mean PTV boost volume was 201 cc and 164 cc in the IMRT and MIBT group, respectively.
Table 1
Patient characteristics
The IMRT boost-related parameters are listed in Table 2A, and the MIBT boost parameters are presented in Table 2B. The mean 95% PTV boost volume received was 99.2% of the prescribed dose, and the mean conformity index (CI) was 0.99 in the IMRT arm. All patients in the MIBT group received a double-plane multicatheter interstitial implant with stainless steel needles. The mean PTV boost volume (V100%) was 85.5%, the mean V90 was 91%, and the mean DNR was 0.32. Supplementary Table 1B provides the number of needles used in double-plane MIBT.
Dosimetry analysis
EQD2 was calculated during both the WBRT and boost phases in both the arms, and the cumulative EQD2 doses are summarized in Table 3. The ipsilateral lung’s cumulative mean lung dose (MLD) was 18 Gy in both the groups, whereas the ipsilateral lung’s cumulative mean V5 was 61% and 62%. The cumulative mean V20 was 39% and 36%, while the cumulative mean V30 was 30% and 28%, respectively. The cumulative mean heart dose (MHD) was 6 Gy in both the arms, the cumulative mean heart V30 was 9% and 12%, the cumulative mean LAD Dmax was 21.5 Gy and 20.1 Gy, and the cumulative mean LAD V15 was 17.69% and 17.36% in the IMRT and MIBT group, respectively. The cumulative mean esophageal dose was 10 Gy in both the arms, the cumulative mean esophagus V35 was 12% and 15%, and the cumulative mean spine Dmax was 36 Gy and 39 Gy in the IMRT and MIBT group, respectively. Nearly 1 Gy was the cumulative mean dose for the contralateral breast, and the cumulative MLD for the contralateral lung in both the arms. However, none of the characteristics showed a statistically significant difference between the two groups. The MIBT arm presented significantly lower cumulative mean skin and rib Dmax (54.3 Gy and 57.4 Gy) than the IMRT arm (62.8 Gy and 64.5 Gy), as shown by a p-value of less than < 0.001.
Table 3
Dosimetry analysis comparing cumulative EQD2 dose (3D-CRT WBRT plus boost)
A focused comparison between the two boost techniques with EQD2 is detailed in Table 4. The IMRT and MIBT arms were compared during the boost phase by administering 0.1 cc, 1 cc, and 2 cc doses to the heart, skin, ribs, ipsilateral lung, contralateral lung, and ipsilateral non-target breast V50 and V90 in both the groups (Table 4). All parameters exhibited statistically significant differences, with p-values < 0.001, except for non-target breast (NTB) V90 and V50.
Table 4
Dosimetry analysis: A comparison between two boost techniques with EQD2 dose
Toxicity
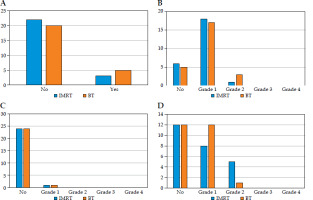
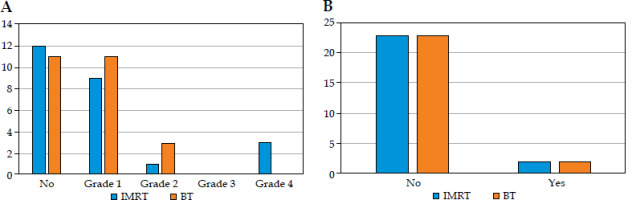
The acute and chronic toxicities are summarized in Table 5A and B, respectively, with the findings further illustrated in bar charts in Figure 4A-D (acute toxicities) and Figure 5A-B (chronic toxicities). 12% of patients in both the arms reported experiencing acute breast pain, while 12% in the IMRT arm and 20% in the MIBT reported having breast tenderness. 72% of patients in the IMRT and 68% in MIBT group had grade 1 acute skin reactions, whereas 4% and 12% had grade 2 acute skin reactions. Both the arms showed 32% and 48% of grade 1 acute dysphagia, and 20% and 4% of grade 2 acute dysphagia in the IMRT and MIBT group, respectively. Both the groups demonstrated 4% of acute pneumonitis, and no patients experienced any cardiac signs or symptoms. There were no statistically significant differences in acute toxicity between the two arms.
Table 5
A) Acute toxicities, and B) chronic toxicities
A
B
In the IMRT patients, 16% of participants reported having chronic breast tenderness, compared with 12% in the MIBT patients, and grade 1 chronic skin reaction was 4% and 0%, respectively. Chronic subcutaneous fibrosis grade 1-2 was observed in 40% and 56% of patients in the IMRT group and MIBT, respectively. Fibrosis of fat necrosis grade 4 was observed in 12% of females in the IMRT group. In the IMRT arm, 8% of females had chronic pneumonitis. In the whole cohort of the study, chronic dysphagia and telangiectasia were not observed, but 8% of patients experienced lymphedema. There were no statistically significant differences in chronic toxicity between the two arms.
Cosmesis
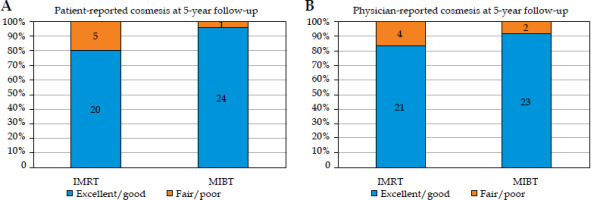
Table 6A and B summarizes the patient-reported and physician-reported cosmetic outcomes. Even though cosmetic outcomes were assessed at 6 months, 1 year, 2 years, and 5 years, the analysis was based on the most mature 5-year follow-up data. No significant variation was noted at earlier time points, as late cosmetic effects typically stabilize over time. The patient-reported cosmetics scored as excellent/good were documented in 80% of the IMRT patients and 96% of the MIBT cases, with fair/poor in 20% and 4% of patients in the IMRT and MIBT group, respectively (p-value = 0.1). The physician-reported cosmetic outcomes scored as excellent/good occurred in 84% of the IMRT patients and 92% of the MIBT cases, with fair/poor in 16% and 8% of patients, respectively (p-value = 0.8). Although there was no statistically significant difference in cosmesis between the two arms, there was no statistically significant difference between patient-reported and physician-reported cosmetic outcomes. However, a non-significant difference was observed, with a higher percentage of patients in the MIBT group reporting excellent or good cosmetics. The overall distribution of cosmetic outcomes is shown in Figure 6A and B (bar charts for patient- and physician-reported outcomes). Supplementary Figure 4A-D demonstrates photographic comparisons of cosmesis between baseline and 5-year follow-up.
Local recurrence and distant metastasis
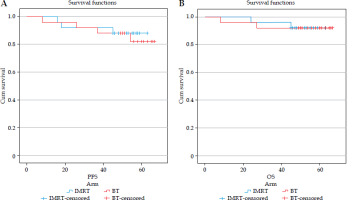
Table 7 summarizes the local recurrence (LR), PFS, and OS, and Figure 7A and B shows the PFS and OS Kaplan-Meier curves. In the IMRT arm, three patients developed LR and distant metastases (DM). One patient experienced TNBC with brain metastases, and died from disease progression. Another HER2-positive patient developed regional lymph nodal and systemic metastases, leading to death. The third HER2-positive patient had extensive metastases (axilla, chest wall, internal mammary, lung, and skeletal), but survived after second-line systemic treatment. In the MIBT group, four patients suffered from a recurrence. Three had TNBC, and one was hormone receptor-positive. One TNBC patient died from brain metastases following interstitial brachytherapy. Another had both LR and DM, and also died. The third patient with brain metastases received Gamma-Knife SRS and systemic chemotherapy; she later developed skeletal metastases, but is alive after third-line treatment. Despite having diffuse metastases, the fourth patient remains alive after receiving treatment with palbociclib, letrozole, and ovarian ablation. The LR was 12% in the IMRT arm and 16% in the MIBT arm, and there was no significant difference between the two arms with a p-value of 1.0.
Progressions-free survival and overall survival
The median PFS was 59 months in the IMRT patients and 61 months in the MIBT cases, with a p-value of 0.7. The median OS was 60 months and 63 months, with a p-value of 0.9 in both the groups, respectively. However, no statistically significant differences in PFS and OS were noted in the two arms.
Discussion
Researchers have studied the dosimetry for 3D-CRT, IMRT, VMAT, SIB, and MIBT boosts as well as toxicity and cosmetic outcomes of hypofractionated whole breast RT, followed by HDR-BT boost. However, no prospective study had thoroughly analyzed dosimetry and its clinical outcomes. The present research is the first to examine these factors in a single study using prospective research.
In a study, Fröhlich et al. [19] used the uniform dose conception (UDC) method to review EBRT with BT boost doses for 24 patients. The author also created additional EBRT boost plans for comparison. Together with 40.05 Gy to the whole breast in 15 fractions, the BT and EBRT to PTV boost dosages were 14.25 Gy over three fractions and 10.7 Gy over four fractions, respectively. The overall dose delivered to PTV in the breast was greater with a BT boost than EBRT. The BT boost administered an increased dosage to the ribs, whereas the skin, lungs, and heart are exposed to reduced levels. Our study also established the cumulative EQD2 dose for both the arms. The only statistically significant difference between the two groups in our study was that the mean cumulative Dmax for the skin and ribs was lower in the WBRT plus MIBT group, leading to better cosmetic outcomes and a lower rate of rib toxicity.
Terheyden et al. [20] compared HDR-BT boost with 10 Gy delivered in one fraction and 3D-CRT boost with 10 Gy in five fractions. They found the HDR-BT boost better at dosimetry for all OARs, leading to less late toxicity. The Dmax for left-sided breast cancers was 29.8% for HDR-BT and 29.95% for EBRT (p = 0.34), the only area where HDR-BT and EBRT did not differ.
Another study by Shahbazian et al. [21] compared MIBT boost with 3D-CRT and electron boost, and discovered that the HDR-BT boost was better at delivering radiation to deep-seated tumors, except for cardiac MHD. Our investigation also showed that the MIBT boost lowers the dose of all OARs better than the IMRT boost, except for the NTB V90 and V50. However, the cumulative dose in cardiac parameters did not differ in both the groups. Supplementary Table 2 presents a comparison of dosimetry outcomes across various research evaluating IMRT and HDR-BT boost techniques.
A retrospective study compared the MIBT and VMAT boosts [22]. Interstitial brachytherapy resulted in decreased doses for the ipsilateral lung (D2cc: 27.6% vs. 73.2%, p < 0.0001), ribs (D2cc: 24.1% vs. 41.2%, p < 0.0001), and the contralateral breast and lung. These results align with the current findings, demonstrating superiority of the MIBT boost in dosimetry over the EBRT boost.
Ciammella et al. [23] performed a study on WBRT using the 3D-CRT technique and a sequential boost using the 3D-CRT technique. They found that the acute grade 1, grade 2, and grade 3 skin reactions were 68%, 15%, and 1%, respectively. The chronic grade 1, grade 2, and grade 3 skin reactions were 18%, 1%, and 0%, respectively. Grade 1 and grade 2 skin fibrosis were 34% and 1.5%, respectively, and grade 3 fibrosis was 0.5%. Our study showed that both the groups exhibited approximately the same number of acute grade 1 skin reactions, with fewer chronic skin reactions. The MIBT patients reported a higher incidence of grade 2 fibrosis in late toxicity, while the IMRT patients showed a higher incidence of grade 4 fibrosis in late toxicity. The grade 4 fibrosis in late toxicity in the IMRT arm may demonstrate the MIBT arm’s cosmesis better, even though there was no statistical difference between the two groups of patients.
Schumacher et al. [24] conducted a retrospective study involving 152 patients, and compared WBRT plus sequential boost IMRT and WBRT plus intra-operative electron radiation therapy (IOERT). Even though 3.2% of patients experienced coughing, 42.1% suffered from dyspnea, and 9.2% had angina as late toxicity, none experienced acute pneumonitis or heart symptoms/signs. The dosimetry advantage of MIBT explains why 8% of the patients in the current study had chronic pneumonitis in the IMRT arm, while no such condition occurred in the MIBT group. Moreover, no cardiac signs or symptoms appeared in any of the patients.
Cardiac issues are most common in left-sided breast cancer patients, with 30% developing an increased risk of cardiovascular issue-related death after 10 years post-radiotherapy [25]; one study found that 1 Gy increased the chance of coronary events by 7.4% [26]. The present study show that the cumulative mean heart dose (MHD) was 6 Gy in both treatment arms, while the mean LAD Dmax was 21.5 Gy and 20.18 Gy, and the mean LAD V15 was 17.69 Gy and 17.36 Gy in the respective groups. No patients in the study developed cardiac problems. However, a 5-year follow-up is insufficient to fully evaluate and confirm this late toxicity to the heart.
Feizi et al. [27] carried out a retrospective (RP) study, monitoring patients for an average of 18 months, and discovered that a higher absolute MIBT breast V29Gy resulted in poorer cosmesis. No patient scored ‘excellent’ in the BCCT core software evaluation, with 32% of poor, 18% of good, and 50% of fair scores. According to Harvard’s scale, 10.5% of patients achieved excellent cosmesis, while 43%, 28.5%, and 18% were categorized as good, fair, and poor, respectively. Contrary to this RP study, another one reported subjective assessment of outcomes as excellent/good in 93% of patients, while objective assessment was reported in 92% of subjects [23]. According to Schumacher et al., the IMRT and IOERT boost techniques did not significantly differ in cosmesis, and the authors concluded that neither treatment was better than the other [24].
Supplementary Table 3 summarizes the toxicity and cosmesis outcomes reported in various published studies. Most available literature reported excellent/good cosmesis achieved in 70-93% and fair/poor in 1.3-18% of patients. The current prospective RCT indicated that the cosmetic outcomes were comparable, with patient-reported excellent/good cosmesis showed by 80% of patients in the IMRT and 96% in the MIBT group, while fair/poor outcomes were reported by 20% and 4% of patients, respectively. Additionally, physician-reported excellent/good cosmesis outcomes were noted in 84% and 92%, and fair/poor outcomes in 16% and 8% of cases, respectively. The study found no significant difference between the two arms in cosmesis, nor between the assessments reported by patients and physician. These results show that WBRT plus IMRT boost and WBRT plus MIBT boost are comparable with regards to cosmetic outcomes.
According to Clark et al. [28] (while the boost arm reduced local recurrence), there was no statistically significant difference in mortality between the radiation with and without a boost. The presented results proved that there was no difference in LRR, PFS, and OS between both the arms.
From a study among 762,468 patients, it was clear that radiotherapy increased the likeliness of patients developing a second cancer, such as lung cancer, esophageal cancer, or sarcoma. The relative risks (RR) were 1.12, 1.39, 1.53, and 2.53, respectively, after five years or more post-treatment. The risk of developing a second lung cancer or esophageal cancer over time at 15 or more years post-breast cancer diagnosis was RR: 1.66 and 2.17, respectively. In the current study, second malignant neoplasm (SMN) did not occur in the participants after a 5-year follow-up. Long-term follow-up is required to assess the incidence of SMN [29]. In both the arms, 8% of patients developed lymphedema, but none developed telangiectasia.
The study is superior because the same patient characteristics were maintained, nearly identical total EQD2 doses were administered in both the groups, ensuring proper follow-up at specific intervals over 5 years. Furthermore, variables that could have thrown off the results were removed, making the study more comparable while increasing its internal validity and decreasing bias. To the best of the authors knowledge, this is the first study to comprehensively compare the dosimetry and clinical outcomes of IMRT and MIBT photon boost following whole breast hypofractionated radiation in post-BCS patients. While the study provides valuable insights, a smaller sample size is a limitation that could affect the generalizability of the results. A larger patient cohort might enhance statistical power, improve the precision of estimates, and provide more robust assumptions.
Conclusions
In the Indian context, boost radiation plays a crucial role following whole breast hypofractionated radiotherapy for majority of patients, who have undergone breast cancer surgery. Dosimetry and clinical outcomes show that interstitial brachytherapy has some benefits over photon boost, including lower cumulative maximum doses to the skin and ribs as well as slightly better long-term patient-reported and physician-reported cosmetic outcomes. Particularly for left-sided breast cancer, image-guided interstitial brachytherapy outscores photon boost due to its superior performance in minimizing cardiac dose. To the authors knowledge, this is the only study addressing this issue in the Asian continent.