Purpose
Lung cancer is among the most prevalent cancers and the leading cause of cancer-related deaths worldwide [1,2]. In China, both incidence and mortality rates of lung cancer had a dramatic increase in the past three decades [3]. Malignant airway stenosis appeared in 20-30% cases of lung cancer, resulting in dyspnea, decreased functional status, and asphyxiation risk [4]. The development of airway stenosis could be associated with intraluminal tumor or compression from outside the tumor [5]. Since surgery was usually not feasible in this situation, palliative intervention was generally utilized to dilate the airway to improve patients’ life quality and the efficacy of anti-tumor therapy. In recent years, various palliative modalities, such as electrocautery, laser therapy, stenting, argon plasma coagulation, cryotherapy, photodynamic therapy, and brachytherapy have been used for relieving airway stenosis [6,7,8,9]. Typically, the choice of therapy was based on the type and location of airway stenosis, pulmonary function, comorbidities, previous treatments, and life expectancy. Recently, 125I radioactive seed implantation had proved its efficacy in various malignant tumors [10,11,12,13]. The results of Lu et al. revealed that trans-bronchoscopy 125I radioactive seed implantation brachytherapy could improve the survival and quality of life of patients with pulmonary atelectasis induced by intraluminal airway obstruction [14]. However, studies on the efficacy of computed tomography (CT)-guided 125I radioactive seed implantation in patients with malignant airway compression (MAC) related to advanced lung cancer are still rare.
This present study aimed to evaluate the feasibility, safety, and efficacy of CT-guided 125I radioactive seed implantation in dilating occluded airway and improving the quality of life in patients with advanced lung cancer related to MAC, due to tumor outside or metastatic lymph nodes.
Material and methods
Ethics
Written informed consent was obtained from all patients. Patients were sufficiently informed of the risks, benefits, and alternatives of 125I brachytherapy. This study followed the ethical guidelines of the 1975 Declaration of Helsinki (revised in Brazil in 2013). This retrospective study was approved by the ethical committee of 3201 Affiliated Hospital of Medical College of Xi’an Jiaotong University, First Affiliated Hospital, Sun Yat-Sen University and Affiliated Cancer Hospital of Guangzhou Medical University.
Patients
From June 2015 to June 2018, a total of 89 patients with stage III-V airway stenosis received 125I radioactive seed implantation in Departments of Interventional Radiology in the three institutions mentioned above. The grade of stenosis was classified as 5 grade scale on the cross-sectional CT imaging area [15]: grade 0 (non-stenosis), grade 1 (1-25% stenosis), grade 2 (26-50% stenosis), grade 3 (56-75% stenosis), grade 4 (76-90% stenosis), and grade 5 (91-100% stenosis). Inclusion criteria for this retrospective study were as follows: 1. Patients diagnosed with primary lung cancer confirmed by pathological examination; 2. Type of airway stenosis confirmed as MAC by both clinicians and radiologists; 3. Patients with at least one complete follow-up record post-implantation. Exclusion criteria for this study were as follows: 1. Patients diagnosed with other tumors (n = 22); 2. Patients diagnosed as primary endotracheal tumor (n = 16); 3. Patients with simultaneous tracheobronchial fistula (n = 7); 4. History of local radiotherapy (n = 4).
125I brachytherapy
The parameters of the 125I seed (CIAE-6711) used in this study was: 4.5 mm length; 0.8 mm diameter and 3.0 × 0.5 mm inside the silver column (adsorption of 125I seed: radioactivity, 0.8 mCi; average energy, 27-35 KeV; half-life, 59.6 days; half layer, 0.25 mm of lead; initial dose rate, 7 cGy/h) and 0.05 mm of external titanium wall.
For targeting areas of interest, 5-mm section from CT images were obtained before 125I brachytherapy. For each patient, a treatment plan was made using a computerized planning system (BT-RSI model TPS; YuanBo, Beijing, China) to determine the dose, number, and locations of radioactive seeds. The prescription dose varied in different institutions, ranging 100-130 Gy.
The implantation was performed in all targeted lesions of treatment section. The brachytherapy applicator’s position on the surface of patient’s body was marked by the preoperative plan based on the treatment planning system. After the induction of local anesthesia (5-15 ml of 1% lidocaine), an 18-gauge applicator was inserted into the lesion and positioned against its deepest margin using CT guidance. The applicator for implantation was attached with a turntable or clip implant gun. A CT scan was performed after implantation to assess whether 125I distribution was consistent with the preoperative plan. If not, a supplemental implantation would be performed. Steroids were not routinely administrated post-implantation. However, if the tumor volume was large and required multiple punctures, or the airway compression was at higher level, steroids, such as dexamethasone, were administrated appropriately post-implantation, usually for no more than three days. In other words, the use of steroids depended on the clinician’s judgment. Three typical cases are presented in Figures 1 and 2.
Fig. 1
Patient with MAC treated with seeds implantation and outcome. A) Preoperative enhanced scanning, location and size of neck mass, grade 4 of compression of trachea, B, C) Pre-TPS showed a peripheral matching dose of 110 Gy, 17 seeds were planned, D) Procedure of 125I seeds implantation, E) 3-month post-implantation, grade 2 of compression of trachea, F) 6-month post-implantation, still grade 2 of compression of trachea, G) 12-month post-implantation, grade 1 of compression of trachea, H) 18-month post-implantation, grade 0 of compression of trachea
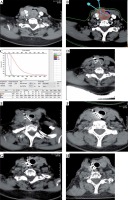
Fig. 2
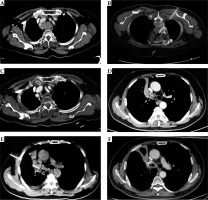
Two patients with MAC treated with seeds implantation and outcome. A) Patient 1: preoperative enhanced scanning, location and size of mediastinum mass, grade 3 of compression of trachea, B) Procedure of 125I seeds implantation, C) 3-month post-implantation, grade 1 of compression of trachea, D) Patient 2: preoperative enhanced scanning, location and size mass in right hilum, grade 3 of compression of right main bronchus, E) Procedure of 125I seeds implantation, F) 1-month post-implantation, grade 1 of compression of right main bronchus
Concurrent therapy
All patients were recommended chemotherapy, targeted therapy, immunotherapy, or even clinical trials of anti-tumor drugs post-125I seed implantation, when meeting indications.
Follow-up
Contrast-enhanced CT images were obtained monthly in the first 3 months post-procedure, and then at 3-month intervals for all patients. Generally, positron emission tomography (PET)-CT scan was performed with the need to evaluate tumor recurrence or metastasis, which could not be found using conventional contrast-enhanced CT image. In addition, all patients were clinically evaluated through history and physical examination at each follow-up visit. Moreover, patients were assessed to determine their Karnofsky performance status (KPS) score. The objective response rate of 125I brachytherapy was based on the Response Evaluation Criteria in Solid Tumors (known as RECIST). In this study, major complications were defined as complication with more than 5% of incidence. Severe complication was defined as life-threatening complication, which needed urgent medical intervention.
Quality of life
The quality of life (QoL) assessment data of all patients were obtained with KPS score. KPS measurements ranged from 0 to 100, with higher score indicating higher QoL. Clinicians determined KPS score prior to CT-guided 125I radioactive seed implantation and at each subsequent follow-up visit.
Statistical analysis
In this study, the decline in stenosis grade of MAC and the local control of tumor, which caused MAC were considered as co-primary ending points of the study, and the other main observations included technological success, safety, and efficacy of 125I seed implantation as well as QoL. Overall survival (OS) was the second observations defined as the time span from the date of 125I brachytherapy implantation to the date of patient’s death from any reason. OS was recorded from the treatment date till the last date of contact. Kaplan-Meier analysis was used to calculate the survival data. Statistical software (SPSS 20.0; SPSS, Chicago, USA) was utilized for performing statistical analysis. P-values less than 0.05 were considered statistically significant.
Results
Patients and procedures
Between June 2015 and June 2018, 40 patients were finally included into the present study, with 28 males and 12 females, median age of 57.9 years. A total of 40 125I radioactive seed implantation sessions were performed. The average number of 125I seeds used per patient was 28.3 (range, 15-45 seeds). The average diameter of the tumor was 4.1 cm (range, 2.8-5.8 cm). Baseline characteristics of all patients are shown in Table 1. The average time of procedure was 66 min (range, 30-90 min). In this study, all interventional procedures were successfully performed and well tolerated. No procedure-associated death occurred, and no patient required an urgent discontinuation of the implantation procedure.
Table 1
Background characteristics of patients before 125I seed implantation (N = 40)
Airway obstruction control
Overall, the stenosis grade of the airway obstruction declined in most patients between the procedure and the last follow-up. At 12 months after the procedure, stenosis grade in 22 (55%) patients became grade 0, while others turn out to be grade 1 (Table 2). The objective response rate (ORR) of local tumor in patients’ survival, calculated as the sum of the complete response (CR) and partial response (PR) was 80%, 100%, 100%, 100%, 87.5%, 83.3% in patients at 1st, 3rd, 6th, 12th, 24th, and 36th months, respectively. There were only 4 patients (10%) with local progress during the follow-up time. Local control of tumor, which caused airway compression is characterized in Table 3.
Table 2
MAC grading pre- and post-125I radioactive seeds implantation in 40 patients with advanced lung cancer
| Follow-up time, months | Patients’ survival, n | MAC grade | |||||
|---|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | V | ||
| 0 | 40 | 0 | 0 | 0 | 14 | 26 | 0 |
| 1 | 40 | 0 | 5 | 10 | 15 | 10 | 0 |
| 3 | 40 | 2 | 6 | 14 | 15 | 3 | 0 |
| 6 | 40 | 10 | 17 | 8 | 5 | 0 | 0 |
| 12 | 40 | 22 | 18 | 0 | 0 | 0 | 0 |
| 24 | 24 | 17 | 4 | 2 | 1 | 0 | 0 |
| 36 | 6 | 5 | 0 | 1 | 0 | 0 | 0 |
Table 3
The efficacy of 125I radioactive seeds implantation for the treatment of malignant airway compression in 40 patients with advanced lung cancer
| Follow-up time, months | Patients’ survival, n | Response of obstruction after 125I seed implantation | |||
|---|---|---|---|---|---|
| CR | PR | SD | PD | ||
| 1 | 40 | 0 | 32 | 8 | 0 |
| 3 | 40 | 2 | 38 | 0 | 0 |
| 6 | 40 | 10 | 30 | 0 | 0 |
| 12 | 40 | 22 | 18 | 0 | 0 |
| 24 | 24 | 17 | 4 | 0 | 3 |
| 36 | 6 | 5 | 0 | 0 | 1 |
Quality of life response
In the majority of patients, KPS scores had improved after implantation, especially in those with a lower KPS score before the treatment. The improvement in symptoms and well-being started in the first month post-procedure and was maintained until the final follow-up in most of the patients. Mean KPS score was 71.4 ±4.8 before surgery and increased to 86.2 ±3.8, 85.9 ±5.1, 84.3 ±4.4, 83.1 ±4.8 at 6, 12, 24, 36 months after the procedure, respectively. There was a significant improvement between pre-procedure and six months post-procedure KPS scores (p < 0.0001), while there was no significant improvement among post-procedure KPS scores.
Survival analysis
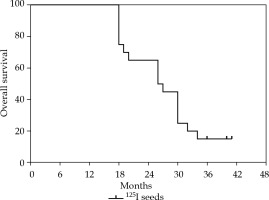
The median follow-up time was 30.3 months (range, 12-41 months), the median survival time was 25.1 months, and the survival rate was 100% at 12 months, 60% at 24 months, and 15% at 36 months after the procedure. At the time of analysis, 34 patients died, and 32 patients died of primary disease progression, including local recurrence and distant metastasis. One patient died of respiratory failure, with a history of COPD, and 1 patient died of myocardial infarction, with a history of hypertension and diabetes mellitus. Outcomes of survival are reported in Figure 3.
Complication
In this study, all patients tolerated the procedure well and no procedure-related death occurred. Complications with an incidence ≥ 10% included irritable cough, temporary hemoptysis, chest pain, fever, pneumothorax, pneumonia, and air leak, which occurred in 26 (65.0%), 31 (77.5%), 12 (30.0%), 15 (37.5%), 11 (27.5%), 6 (15%), and 4 (10%) patients, respectively. One patient suffered from chest pain for more than a week, with an increased leucocyte level and was suspected of mediastinitis; the complication was resolved with antibiotics administration. No patients required additional emergency intervention treatment. Detailed information is shown in Table 4.
Discussion and conclusions
Malignant airway stenosis presented in late-stage lung cancer has a dismal long-term survival, with < 1% of five-year survival rate [1]. Systemic chemotherapy considered as standard therapy for late-stage lung cancer could be unbearable for patients with poor performance status and not provide immediate recanalization for airway stenosis [16]. Therefore, external beam radiation therapy (EBRT) is recommended as palliative treatment in such cases. However, the result of EBRT is usually unpredictable, with possible aggravation of airway obstruction related to early tumor edema [17]. Recently, a variety of bronchoscopic techniques are available for the treatment of airway stenosis [5,6,7,8,9]. Unfortunately, there is a high, 24% rate of stent re-stenosis within 3 months due to in-growth of initial malignancy. Moreover, a placement with stent re-stenosis offers limited survival benefits in the absence of locoregional therapy for endobronchial lesions. Recently, a novel therapy with 125I seed was developed and exhibited promising outcomes in lung cancer [10,12,13]. In the present study, we also confirmed that CT-guided 125I implantation was safe and efficient in MAC patients.
In low-dose-rate (LDR) therapies, brachytherapy delivery requires < 1 Gy per hour for permanent placement of radioactive sources, while in high-dose-rate (HDR) therapies, radiation is delivered over several courses [18]. In previous reports with HDR brachytherapy, isotope iridium-192 (192Ir) was used for airway malignancy, with dose regimens ranging from 4 treatments with 5 Gy each to 2 treatments with 15 Gy each [19]. However, in this study, 125I seed was employed as brachytherapy source, with a prescribed dose of 100-130 Gy. Moreover, intraluminal HDR brachytherapy to a certain extent was not suitable for patients with MAC from outside the tumor. Continuous LDR brachytherapy with 125I seeds could contribute to an increase in radiation damage of radiosensitive G2-M phase tumor cells [20]. Furthermore, LDR brachytherapy with 125I seeds allows for efficient repair of normal tissue with sublethal radiation damage [21]. In the present study, the technical success rate of 125I seed implantation was 100% and no 125I seed loss occurred in the delivery. During the follow-up time, the patients tolerated 125I seed implantation well, which could be due to a reasonable choice of isotope, sophisticated design of TPS plan system, and precise technique of implantation presented by experienced interventional radiologists.
Objective response rates of targeted lesion were 100%, 100%, 100%, 87.5%, and 83.3% at 3rd, 6th, 12th, 24th, and 36th months post-procedure, respectively. Compared with a previous study [19], recanalization obstruction in our study demonstrated similar satisfying effects with longer duration. Possible reason could be due to more sustainable therapeutic effect of 125I radioactive seed implantation. The majority of patients in the present study showed improved KPS score after the implantation procedure. The mean KPS score was 71.4 ±4.8 before procedure and improved to 86.2 ±3.8 at six months after implantation (p < 0.0001). The sustaining therapeutic effect of 125I radioactive seed implantation could be responsible for the comparatively long duration of KPS improvement.
The median survival was 25.1 months, and the survival rates were 100% at the 12th month, 60% at the 24th month, and 15% at the 36th month. The two-year survival rate was higher in the current study compared with a previous study with HDR intraluminal brachytherapy [22]. The difference may result from patients’ selection or additional therapies, such as chemotherapy, immunotherapy, and targeted therapy. Overall, 60% 2-year survival rate was a favorable outcome for the present study, in which most patients presented with advanced stage disease.
While complications of HDR intraluminal brachytherapy were generally moderate, severe complications, including fatal hemoptysis and broncho-esophageal fistula were still occasionally observed [23,24]. Compared with HDR, LDR presented better relative biological effect (RBE) [25,26]. In the present study, treatment complications observed were mild, mainly presenting as a temporary increase in irritable cough, transient hemoptysis, chest pain, fever, and pneumothorax, which were resolved with few additional therapies. No severe complications were noted. Therefore, 125I seed implantation brachytherapy could be a preferable treatment option with regards to complications and could be repeated whenever indicated [27].
This study had several limitations. Firstly, it was a retrospective non-control study, and cautious interpretation of the results are needed. Secondly, affected by the experience of operating interventional radiologists in different institutions, the precise positioning of radioactive seed designed by a preoperative plan of TPS was occasionally challenging. Lastly, the current study involved small cohort of patients, which undermine the conclusion.
In conclusion, the implantation of 125I seeds can be a safe and effective alternative therapy for patients with inoperable MAC. It presents excellent local control efficacy, with moderate side effects and acceptable tolerability. Nevertheless, a comprehensive evaluation of the long-term effects of 125I seed implantation treatment is still needed.