Purpose
Lung cancer is the second most common cancer worldwide [1], and is generally categorized into small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC), according to the morphology of the cancer cells [2]. SCLC is an aggressive epithelial tumor characterized by a high propensity for early development, which accounts for about 15% of all lung cancers [3, 4]. Approximately, two-thirds of SCLC patients develop metastatic diseases with limited treatment options, suggesting that SCLC remains one of the most lethal malignancies [2]. Radiotherapy and chemotherapy are the cornerstones of SCLC treatment, and a combination of immune checkpoint inhibitors can also be employed to treat extensive SCLC [3, 5]. Etoposide and platinum-doublet chemotherapy is recommended as the standard first-line therapy, and combination chemotherapy with other agents, including carboplatin (paraplatin) and irinotecan (camptosar), is considered the standard second-line treatment [4, 6]. In patients with limited-stage SCLC (LS-SCLC), the combination of chemotherapy and radiotherapy may improve local control as well as overall survival (OS). However, for patients with extensive-stage SCLC (ES-SCLC), who make up the majority of SCLC patients in clinical practice, the most common initial chemotherapy regimen remains etoposide plus cisplatin (EP regimen), and no novel chemotherapy combinations have shown superior efficacy [3, 6]. Therefore, there is an urgent need for a safe and effective treatment to improve the clinical prognosis of SCLC patients.
Iodine-125 (125I) radioactive seed implantation therapy is a local treatment that has been widely used in treatment of various malignancies [7-9]. With the advantages of low doses, small adverse reactions, and minimal invasiveness, it has been increasingly applied in treating NSCLC, achieving certain efficacy [10-12]. However, this method is rarely used in SCLC treatment. Therefore, this study retrospectively analyzed data of SCLC patients treated with 125I seed implantation, and evaluated its’ efficacy and the corresponding complications.
Material and methods
Patient data
A total of 12 SCLC patients, who were admitted to the respiratory and critical care medicine department of our hospital from September 2014 to September 2018 were enrolled into this study. Inclusion criteria: (1) Patients aged ≥ 18 years; (2) Diagnosed with SCLC by fiberoptic bronchoscopy or percutaneous lung biopsy; (3) Those who received 125I radioactive seed implantation. Patients with respiratory failure, heart failure, severe arrhythmia, severe emphysema, high-risk of bulla puncture, and severe coagulation dysfunction as well as patients with incomplete data or follow-up information were not considered for this retrospective analysis. Clinical characteristics of the patients at baseline are shown in Table 1. Characteristics of each patient are presented in Tables 2 and 3, which included TNM stage, tumor location and size, prescription dose, previous treatment, and reasons for omitting standard treatment.
Table 1
Patients’ characteristics
Table 2
Characteristics of patients with 125I seed implantation
Table 3
Previous treatments of patients with 125I seed implantation and reason for omitting a standard treatment
Instruments and equipment
For 125I radioactive seed implantation, 18 G implantation needles and a turntable implantation gun were obtained from HAKKO Medical Co., Ltd. (Nagano, Japan) and Zhuhai Hejia Medical Equipment Co., Ltd. (Zhuhai, China), respectively. 125I seeds were produced by Beijing ZHIBO Bio-Medical Technology Co., Ltd. (Beijing, China), and were fully enclosed in a titanium shell, with a length of 4.5 mm, a diameter of 0.8 mm, a half-life of 60.1 d, and an activity of 0.7 mCi. Seed implantation was guided by a Siemens 32-row helical computed tomography (CT) scanner (Siemens, Erlangen, Germany), with a slice thickness of 5 mm.
Pre-operative preparation
Before 125I seed implantation, patients underwent a blood routine examination, accompanied by withdrawal of antiplatelet and anticoagulant drugs. An enhanced chest CT examination was performed to clarify the lesion scope and its’ relationship with blood vessels as well as the route of needle insertion. Then, treatment planning system (TPS; Beijing Tianhang Kelin Technology Development Co., Ltd., Beijing, China) was applied to determine the number and distribution of 125I seeds. Prescribed dose was 100-120 Gy. Prior to implantation, codeine was given to relieve cough, and diazepam was applied for sedation according to a patient’s condition. Those with a high-risk of bleeding were administered hemostatic drugs, such as intra-muscular injection of hemagglutinin, and intravenous access was established.
CT-guided 125I radioactive seed implantation
Patients were placed in a supine, prone, or lateral position, depending on the lesion location. Oxygen inhalation and ECG monitoring were given during the operation, and CT scans were conducted to determine the tumor site and the needle insertion point of 125I seeds, with a line spacing of 1-1.5 cm and particle spacing of 0.5-1 cm. After routine skin disinfection with iodophor, a sterile hole towel was laid, and the puncture point area was anesthetized with local infiltration of 2% lidocaine. The syringe needles were indwelled after local anesthesia at all puncture points. Next, a CT scan was performed to examine whether the puncture point and puncture angle were correct. Then, the syringe needle was pulled out, and the implant needle was punctured at a suitable point and angle. After confirming the correct puncture location by a CT scan, 125I seeds were implanted and post-operative verification was conducted. Subsequently, the needle was removed, and the puncture point was covered and fixed with sterile gauze and tape. If there was a bleeding from the puncture point, compression was carried out.
Outcomes and assessments
Post-operative complications were closely monitored, and all patients were reviewed every 2 months. Patients were followed up until death, last visiting time, or the end of study. The efficacy was evaluated using response evaluation criteria in solid tumors (RECIST) guideline, version 1.1 [13]: (1) Complete response (CR): All target lesions disappeared and short-axis of any pathological lymph node (whether target or non-target) must be less than 10 mm; (2) Partial response (PR): A reduction of at least 30% in the sum of target lesion diameters compared with that of baseline sum diameters; (3) Progressive disease (PD): At least 20% of an increase in the sum of target lesion diameters compared with the smallest sum in the study (including the baseline sum if that was the smallest in the study). In addition to the relative increase of 20%, the absolute increase in the sum of diameters must be greater than 5 mm. The appearance of one or more new lesions was also considered progression; (4) Stable disease (SD): Neither sufficient shrinkage to qualify for PR nor enough increase to qualify for PD, the smallest sum diameters were used as a reference for the study. Overall response rate (ORR) = (CR + PR)/total number of cases × 100%. Local control rate (LCR) = (CR + PR + SD)/total number of cases × 100%. OS was defined as the time from the start of treatment to the death of a patient due to any cause. Progression-free survival (PFS) was considered the period between implantation and the date of recurrence, loco-regional progression, metastasis, or death.
Results
Characteristics of iodine-125 seed implantation
All patients were successfully implanted with 125I radioactive seeds. As depicted in Table 2, a total of 662 125I seeds were implanted into 19 tumor lesions among the 12 patients, with a range of 20-89 seeds implanted in each patient. One of the cases 9 was found to have a dose cold zone, localized, with a supplemental implantation of 13 seeds.
Efficacy
The ORR was 83.3%, 63.6%, 50%, and 40% at 2, 6, 12, and 24 months after implantation, respectively (Table 4). The LCR at 1 and 2 years was 75% (6/8) and 60% (3/5), respectively. The patient’s treatment results are shown in Figure 1. Of the 12 patients, 2 patients with superior vena cava syndrome had complete remission, and 2 of the 3 patients with atelectasis had lung recruitment.
Table 4
Efficacy evaluation of 125I radioactive seed implantation
| Time after surgery (months) | n | CR | PR | SD | PD | ORR (%) | LCR (%) |
|---|---|---|---|---|---|---|---|
| 2 | 12 | 4 | 6 | 2 | 0 | 83.3 | 100.0 |
| 6 | 11 | 3 | 4 | 3 | 1 | 63.6 | 90.9 |
| 12 | 8 | 2 | 2 | 2 | 2 | 50.0 | 75.0 |
| 24 | 5 | 1 | 1 | 1 | 2 | 40.0 | 60.0 |
Fig. 1
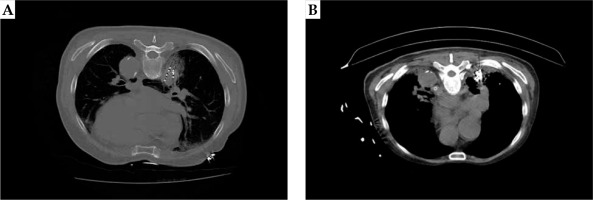
CT images showing particle distribution after 125I radioactive seed implantation in small-cell lung cancer (SCLC) patients and treatment after 3 months. A) After 125I radioactive seed implantation, a few bleeding complications appeared in the dorsal lung of the tumor. B) Three months after 125I radioactive seed implantation, the tumor gradually shrunk, and the gathered particles could be seen at the original tumor site: mild radiation pneumonia in the lung tissue around the tumor
Survival analysis
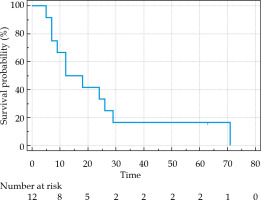
As shown in Figure 2, the patients who received 125I seed implantation had a median OS of 12 months (range, 5-71 months). Moreover, the OS rate at 6, 12, and 24 months after implantation was 91.67%, 66.67%, and 41.67%, respectively (Table 2). The median PFS was 8 months (range, 3-69 months).
Discussion
Iodine-125 seed implantation therapy was first used in the treatment of prostate cancer, and with the continuous innovation and improvement of medical technology, it is now widely used in treating various malignancies. Its’ efficacy has been fully verified in the treatment of NSCLC [10-12]. A meta-study on NSCLC showed significant differences in ORR, disease control rate (DCR), and OS between 125I brachytherapy in combination with chemotherapy and chemotherapy alone, confirming the clinical benefit of 125I seed implantation for NSCLC treatment [14]. Additionally, lung squamous cell carcinoma is sensitive to 125I seed irradiation, which exhibits significant therapeutic effects. However, there is little information on other pathologic lung tumor types irradiated by 125I seed irradiation in addition to NSCLC [15]. In the current study, 12 SCLC patients received 125I seed implantation, and it was found that tumor growth was inhibited and the quality of life improved. The 125I radioactive particles can continuously release low-dose γ-rays to break molecular chains and chemical bonds of biological macro-molecules, thereby destructing DNA, RNA, proteins, and various catalytic enzymes, with no or only slight damage to normal tissues. In addition, in the tumor micro-environment, 125I irradiation inhibits cell proliferation by reducing the level of Ki-67, and induces apoptosis by increasing the P21 level and reducing the surviving level in vivo [15]. Therefore, inhibition of cell proliferation and induction of apoptosis by 125I irradiation is the key mechanism for the therapeutic effect of 125I seed implantation [16].
Unlike other types of lung cancer, SCLC is initially sensitive to chemotherapy and radiotherapy. However, despite the sensitivity to radiotherapy and chemotherapy, patients with newly diagnosed extensive-stage SCLC generally have a poor prognosis, with a median PFS of 5.5 months and a median OS of 10 months [17]. According to a previous study, the low OS rate in highly chemotherapy-sensitive SCLC populations is due to the rapid development of drug resistance and the failure of second-line and follow-up treatments to affect or slow tumor growth and metastasis [18]. For decades, both ESMO [6] and NCCN [4] clinical treatment guidelines have recommended chemotherapy containing EP as the standard treatment for ES-SCLC. An earlier paper reported that the average response rate of patients treated with the EP regimen ranged from 60% to 80%, with a median OS of 8-10 months [19]. In this research, patients receiving 125I seed implantation had an ORR of 83.3%, 63.6%, 50%, and 40% at 2, 6, 12, and 24 months, respectively. Furthermore, the median PFS was 8 months, the median OS was 12 months, and the mean OS was 23.6 months, which was superior to the efficacy of standard treatment. In addition, a randomized phase 3 CREST trial published in 2015 [20] evaluating thoracic radiotherapy for ES-SCLC, revealed that 247 patients who received thoracic radiotherapy (30 Gy in ten fractions) had a 1-year OS rate of 33% and 2-year OS rate of 13% as well as a 6-month PFS rate of 24%. In this study, the incidence of OS at 12 and 24 months after the surgery was 66.67% and 41.67%, respectively, which was higher than that of ES-SCLC patients, who received general systemic treatment or chest radiotherapy after chemotherapy [20]. Nevertheless, more studies are still needed to compare thoracic radiotherapy and high-dose-rate brachytherapy (HDR-BT) with 125I seeds brachytherapy for the treatment of SCLC. However, HDR-BT lacks experience and data from large phase III clinical trials in lung cancer, since most studies have focused on NSCLC, with few data on SCLC. In addition, the quality control of HDR-BT technology is difficult to be guaranteed due to the characteristics of personal proficiency and individualized operation techniques.
Although 125I seed implantation has the advantages of minimal invasiveness, quick recovery, and few adverse reactions, there are various complications, such as worsening cough, pneumothorax, and hemoptysis. Generally, patients who cannot tolerate radiotherapy or chemotherapy and refuse radiotherapy and chemotherapy, present with tumor emergencies, and whose disease has progressed after radiotherapy and chemotherapy, could benefit from 125I seed implantation therapy. However, for SCLC, 125I seed implantation is the only local treatment, and systemic treatment is still required.