Purpose
Uterine and vaginal malformations due to abnormal development of the Müllerian ducts are classified as non-syndromic minor malformations. These include those found during systematic examination for infertility as well as genital organs that are completely blocked, with early symptoms during the menstrual period. Although it is difficult to determine the frequency of uterus didelphys accurately, which varies according to reports, the frequency has been reported to be about one in 10,000 (range, about 1 in 1,000 to 1 in 30,000) of obstetric admissions in general [1,2]. There is no standard treatment for cervical cancer occurring in uterine malformations. We report a rare case of cervical cancer that occurred in uterus didelphys (with double vagina and uterus), cured with concurrent chemoradiotherapy (CCRT).
Case report
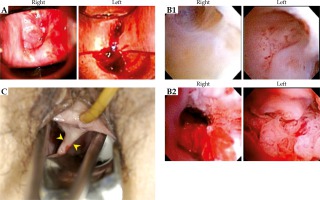
The patient was a 61-year-old woman, gravida 0, para 0. She was referred to the Hiroshima University Hospital on October 19, 2011, with atypical genital bleeding in the last 4 months. She had been diagnosed in our hospital to have uterus didelphys with double vagina and uterus at the time of examination for infertility at the age of 29. Her past history revealed previous surgery for hip osteoarthritis at age of 20. Her family history was unremarkable. The patient was found to have normal vulva. No renal or any other abnormalities were present. Several pelvic examinations in our hospital showed cervical tumor in the uterus didelphys, with a complete vaginal septum extending to the introitus. However, the uterus didelphys had not been observed by the referring hospital, but initially at our hospital. Because the patient complained of pain during pelvic examination, biopsy was performed under intravenous anesthesia, and the initial pathological result of a cervical tumor in one cervix was CIN 3. Furthermore, pelvic examination, hysteroscopy, and biopsy were performed under intravenous anesthesia in the operating room. At that time, the gynecologic oncologist observed the uterus didelphys with vaginal septum for the first time. There were easy bleeding tumors on each cervix at Cusco’s examination. Macroscopic findings, hysteroscopic findings, and the vaginal septum at BT are shown in Figure 1. The tumor at the left cervix was circumferential, whereas the tumor at the right cervix had a left side deviation (septum side) at the hysteroscopy. Histopathological findings of the bilateral cervical biopsy specimens indicated non-keratinizing squamous cell carcinoma. Human papillomavirus (HPV) infection was not investigated.
Fig. 1
A) Macroscopic findings of the right and left uterine cervices at Cusco’s examination. B) Findings on hysteroscopy. B-1) Uterine body (right and left): Each tumor had not invaded the uterine body. B-2) Uterine cervix: The left tumor is larger than the right one. The tumor at the left cervix was circumferential, the tumor at the right cervix was on the left side of the deviation. C) Bilateral vagina with ovoid applicator insertion. These two arrowheads point to the vaginal septum extending to the introitus
Initially, in our department, sounding of the right uterus was impossible, while the sounding length of the left uterus was only 2 cm. The tumor on the left side was larger than that on the right side, and pelvic examination revealed that the left side tumor had invaded the left parametrium. No invasion was observed in either of the vaginal walls.
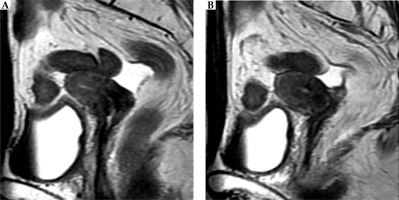
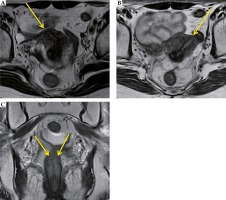
Positron emission tomography-computed tomography (PET-CT) of the head to the pelvis, CT of the chest to the pelvis, and magnetic resonance imaging (MRI) of the pelvis were performed. These showed a tumor with a maximum diameter of 43 mm (more to the left side), spread across the bilateral uterine cervices, and without pelvic and paraaortic lymph node enlargement. According to the Fédération Internationale de Gynécologie et d’ Obstétrique (FIGO) classification [3], the tumor was stage IIB. The serum level of the tumor marker, squamous cell carcinoma (SCC) antigen was elevated to 7.7 ng/ml (normal range, ≤ 1.5 ng/ml). The serum level of the tumor marker, carcinoembryonic antigen (CEA), and blood counts were within the normal ranges. The pre-treatment MRI images of the tumor in bilateral uteri are shown in Figure 2. The tumor on the right side of the uterus was localized in the cervix. On the other hand, the tumor on the left side showed extrauterine invasion (Figure 2). Figure 3 shows the MRI before and during treatment, from bilateral uterine bodies to fundus and vagina.
Fig. 2
Pelvic magnetic resonance imaging (MRI) before treatment. A-1) Right uterine tumor is confined to the cervix (arrow). A-2) Tumor of the right cervix. B-1) Left uterine tumor invades to the surrounding tissue beyond the cervix. B-2) Tumor of the left cervix
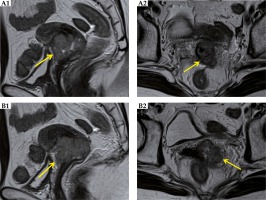
Fig. 3
MRI before and during treatment. A) Right uterine body to the fundus. B) Left uterine body to the fundus. C) Bilateral vaginas
In addition to the carcinoma of the uterus and vaginal didelphys, based on the pelvic examination and imaging diagnosis, the tumor invasion range in the patient was considered to be wide (stage IIB, with left parametrial involvement, tumor size of 43 mm). This finding was judged to be an indication for radiotherapy rather than surgery. The type of uterine didelphys in the patient according to Jarcho classification [4] was categorized as class 1. Uterus didelphys bicollis (septate vagina) according to the American Society of Reproductive Medicine classification [5] was class III. Radiotherapy (RT) was administered via a combination of external beam radiotherapy (EBRT) to the pelvic cavity and intracavitary irradiation. EBRT was given to the whole pelvis using four-field box technique with a 10-MV linear accelerator unit. The daily fraction size was 2.0 Gy, with 5 fractions weekly. Details of the radiation field were reported previously [6]. A central shield was used after 40 Gy, with external whole pelvic irradiation. From November 22 to December 29, 2011, the total dose administered was 50 Gy (25 fractions/38 days). High-dose-rate intracavitary BT (HDR-ICBT) was given with 192Ir micro-Selectron (Nucletron, The Netherlands). The dose at point A was 6 Gy per fraction, 1 fraction per week, and the number of fractions was 4, for a total dose of 24 Gy on December 20 and 27, 2011 and January 4 and 10, 2012. Total treatment time of RT was 50 days. With the cooperation of the gynecological oncologists, ultrasound guidance was used, and it was confirmed that the tip of the tandem reached the fundus of the uterus. Because each vagina was very narrow and the patient complained of pain at the time of application, all 4 applications were performed under intravenous anesthesia. Oxygen was administered at 3 liters per minute with an oxygen mask. At the time of application, vaginal packing with gauzes containing radio-opaque fiber were used to show the state and thickness of the rectal and bladder sides packing. Tandem and ovoid applicators were used for each application. The ovoid size was 30 × 20 × 14 mm in diameter. At the first treatment, only the left side of the uterus was treated with tandem and ovoid applicators. Because the vagina was very narrow, we could not interpose the flange with the two ovoid applicators (these two applicators deviated to the foot side from the flange). The sounding length to the fundus of the left uterus was only 3.5 cm. At the second to third treatments, a tandem was inserted to the left uterus, whereas two ovoid applicators were placed at the right and left vaginal fornices. By the second time, tandem insertions into the uterine cavities on both sides were attempted. The sounding length of the right uterus was 4 cm. However, when a tandem was first inserted on the right side, the insertion on the left side became insufficient because of the narrowing between the bilateral cervical canals. Therefore, a tandem was inserted only on the left side containing the large tumor. We were worried about the insufficient dose to the whole uterus on the right. Therefore, at the last treatment, a tandem and a right-side ovoid were inserted to each of the right uterus and the right vagina, respectively, and a left ovoid was inserted to the left vagina. Dose distributions of each ICBT are shown Figure 4 [7]. Although it is an approximation, most of the tumor volumes before treatment with each ICBT (red dashed lines drawn by referring to the pre-treatment MRI image) are surrounded by 6 Gy isodose curves. In addition, the tumor volume just after the second ICBT (yellow dashed line drawn by referring to MRI just after the second ICBT) was completely enclosed within the 6 Gy isodose curve.
Fig. 4
Dose distributions of each of the four brachytherapy. The small schemas on the upper left side of the figures were drawn, modified using reference [7]
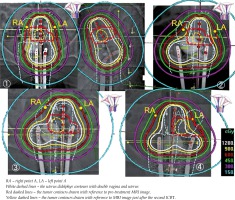
The distances between the bilateral ovoid sources at each application were 1.2, 3.5, 3.6, and 3.2 (mean, 2.9) cm, respectively. We regarded the thicknesses of X-ray gauze packing as the distance between ovoid and vaginal posterior wall for each ICBT. Those measurements were 0, 4.5, 2.6, and 0 (mean, 1.8) mm. Orthogonal X-rays were used for the calculation of these distances.
The rectal dose was evaluated using a rectal dosimeter, and the reference dose point as well as the bladder dose were defined according to the recommendations of the ICRU 38 report [8]. There were five measurement points based on the rectal dosimeter, and the maximum one was evaluated. The mean doses at point A, at the rectum by the ICRU 38, at the rectum by calculation, at the rectum by dosimeter measurement, and at the bladder by the ICRU 38 per fraction were 6.1 Gy, 7.7 Gy, 5.2 Gy, 4.2 Gy, and 7.8 Gy, respectively. The total doses of these five points were 72.7, 107.9, 74.1, 64.0, and 107.6 GyEQD2, respectively. The patient received six courses of concurrent chemotherapy, with 30 mg/m2 of weekly cisplatin (CDDP). No acute adverse events such as diarrhea occurred in this patient.
The primary tumor response was the complete response (CR) by pelvic examination just after CCRT; and by pelvic images on MRI and CT at 4 months after CCRT (Figure 5). The serum SCC level returned to within normal limits, with a value of 0.8 ng/ml.
Four years and three months after CCRT, the patient developed a late rectal complication, grade 1 radiation proctitis, which was graded according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer late radiation morbidity scoring criteria [9]. The patient has been followed-up without treatment because she has no anemia. The patient was alive and well with no evidence of recurrence or any other late complications 6 years and 8 months after the start of CCRT.
Discussion
It is very rare to encounter uterine malformations in daily gynecologic cancer examinations. The treatment of a patient with carcinoma of the cervix in a uterus didelphys is not described or established in textbooks, but it is only seen in case reports. The individual patient treatment is determined by the judgment of each physician. Therefore, we believe that it is meaningful for such physician to report the treatment method. Then, if physicians encounter similar cases, they can discuss the treatment method whether radiotherapy is possible and how to irradiate concretely. These actual experiences with cases are very valuable, and the physicians can refer to them in the literature. Patients can be treated similarly based on these experiences and planned for further treatment. If these cases are accumulated, the treatment method for a patient can be established. We can then compare radiotherapy (RT) with surgery by referring to the following surgical cases. Several reports of carcinoma in a uterus didelphys, which were treated surgically with or without preoperative RT were recognized until now, including a case where one side was non-cancerous, while the other side had cervical or endometrial cancer [10,11,12,13,14,15,16,17,18]. In total, there were only seven radiotherapy cases of cervical cancer in uterus didelphys reported until now (Table 1). It is very important to discuss the treatment method whether RT or surgery for the uterus didelphys at diagnosis.
Table 1
Case reports of patients with uterine anomaly treated with brachytherapy
| No. | Age (yr) | FIGO | Path | Uterine anomaly (Class) | EBRT | CS | BT: Reference point, dose-rate, applicator, dose | EQD2 | Concurrent chemo. regime | RFS mo | Author, year |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | II | SCC | Uterus didelphys with vaginal subseptum (III) | Point B; SP 3000-6000r : Deep X-ray | N | Point A; 7000 r, LDR, 1588 mgRa, 2 intrauterine tubes + 2 ovoids | – | N | 12 | Gauwerky F, 1955 [19] |
| 2 | 45 | IIA1 | L: SCC R: CIN 3 | Uterus didelphys with double vagina (III) | WP 45 Gy/25 fr | N | Modified point A, HDR, 2 tandems + 2 cylinders, 6 Gy × 1, 6.5 Gy × 1 | PA 60.8 | Unknown | 36 | Lee CD, 2000 [20] |
| 3 | 58 | IIA2 | SCC | Bicornuate uterus (IV) | WP 50 Gy/25 fr | N | Defined point A, LDR, flexible intrauterine catheter (r × 1, l × 1) + cylinder, 9 Gy × 2 | – | w CDDP, 40 mg/m2 × 5 | 24 | Loo HW, 2010 [21] |
| 4 | 34 | IIB | AD | Septate uterus (V) | WP 45 Gy/25 fr, LN boost 9 Gy | N | Point A, HR-CTV, HDR, rotte + 2 ovoids, 5.5 Gy × 5 | CTV 86.3 | w CDDP, 40 mg/m2 × 6 | 20 | Platta CS, 2014 [22] |
| 5 | 37 | IIIA | AD | Uterus didelphys with vaginal simplex (III) | Ext. field 50.4 Gy/28 fr, GTV 60 Gy/28 fr | N | HR-CTV, PDR, vaginal mold, 20 Gy, 0.5 Gy/hr × 40 pulse | CTV 76.5 | w CDDP, 40 mg/m2 × 5 | 30 | Cordoba A, 2017 [23] |
| 6 | 33 | IIB | SCC | Septate uterus (V) | WP 45 Gy/25 fr | N | HR-CTV D90, HDR, tandem in RT side + 2 ovoids, 28 Gy/4 fr | CTV 84.4 | w CDDP, 40 mg/m2 | – | Yavas G, 2017 [24] |
| 7 | 55 | IIIB | SCC | Septate uterus (V) | WP 50 Gy/25 fr | 30 Gy | Point A, HR-CTV, tandem in the RT side + 2 ovoids, 6 Gy × 4 | CTV 61.8 | w CDDP, 40 mg/m2 × 5 | 1.5 | Ishibashi N, 2018 [25] |
| 8 | 61 | IIB | SCC | Uterus didelphys with double vagina (III) | WP 50 Gy/25 fr | 40 Gy | Point A, HDR, tandem (l × 3, r × 1) + 2 ovoids, 6 Gy × 4 | PA 72 | w CDDP, 30 mg/body × 6 | 80 | Present case |
[i] Path – pathology, EBRT – external beam radiation therapy, CS – WP dose up to the central shield, EQD2 – 2 Gy per fraction-equivalent dose, regime – regimen, RFS – recurrence-free survival, mo – months, SP – small pelvis, L – left, R – right, SCC – squamous cell carcinoma, AD – adenocarcinoma, WP – whole pelvis, N – none, PA – Point A, LN – lymph node, BT – brachytherapy, fr – fractions, GTV – gross tumor volume, HR-CTV – high-risk clinical target volume, HDR – high-dose-rate, LDR – low-dose-rate, PDR – pulsed-dose-rate, w CDDP – weekly cisplatin
The diagnosis of cervical cancer in uterus didelphys with vaginal septum is very difficult; however, very important. When the cervical cancer of the cervix is identified in only one side, leaving the other side unidentified, the dose for the other tumor and uterus may be insufficient, because only one uterus would receive ICBT. Thus, in a patient with such inadequate dose prescribed for the other cervical cancer, a risk of recurrence may occur. A sufficient inquiry during medical examination of a patient is particularly important for the diagnosis of cervical cancer in uterus didelphys. There are uterine malformations, which adopt an autosomal recessive or dominant genetic form. The uterus didelphys may complicate urinary system malformations, skeletal system, and auditory diseases among others. According to a report, in 5-10% of infertile patients, uterus didelphys is implicated. With a history of infertility or nulliparity, with malformations mentioned above (in a patient or her family members), we should carefully conduct a pelvic examination of the patient, with the existence of a uterus didelphys in mind. This current case was also an infertile patient, with complications of the hip osteoarthritis. Moreover, in a case with a complete vaginal septum extending to the introitus, as in this patient, we may not notice the existence of another vagina and uterus without careful observation of the vulva and vagina before Cusco’s examination. The practical clinical point in the management of a patient with abnormal bleeding that the finding of a vaginal septum impels careful search for a second cervix has been emphasized [17].
As with this patient, the uterus didelphys was diagnosed at the time of examination for infertility. If the patient had not undergone scrutiny for infertility, the uterus didelphys might not have been discovered. In that case, without careful internal examination by a physician, uterus didelphys would not have been diagnosed.
The role of HPV infection has become clear in cervical cancer occurrences. The role of transmissible coital factors in the causation of invasive carcinoma of the cervix have been reported [11,12,14]. In our case, two possibilities of carcinogenesis are conceivable. First, bilateral uterine cervix was exposed during sexual intercourse, with each individually developing uterine cervical carcinoma. Second, only one side of the uterine cervix was exposed during sexual intercourse, and the tumor that developed on one side invaded the other side. If we had examined for the HPV status of the bilateral uterus, and each type was different, there was the possibility that they might have been experienced carcinogenesis separately. However, since mixed infections do exist, the actual mechanism of the carcinogenesis of the bilateral uterus may be unknown.
Surgery was judged to be difficult, because the present patient had a stage IIB locally advanced cancer, which was larger than 40 mm in size, with left parametrium invasion. It was difficult to diagnose whether the right and left tumors were independent [10]. The left tumor was larger than the right tumor at pelvic examination, in hysteroscopic findings, and at MRI. Thus, there is the possibility that the tumor on the left grew in continuity and invaded the right side of uterine cervix.
As mentioned above, to the best of our knowledge, there were only seven previously reported cases of carcinoma of the cervix in patients with uterine anomalies treated with radical radiotherapy (Table 1). In six institutions in countries other than Japan, central shields were not used. For the first time, Gauwerky reported BT as a treatment for FIGO stage II SCC of the cervix with uterus didelphys with vaginal subseptum [19]. Two intrauterine tubes and 2 ovoids were used for double uterus and bilateral vaginal fornix. Lee et al. reported BT as a treatment for FIGO stage IIA1 SCC of the cervix with uterus didelphys, class III Müllerian duct anomaly [20]. Instead of using the traditional point A, the prescription point was 2 cm superior to the mean position of the small metallic flange located at each cervical os at the midline between the two intra-uterine tubes. Loo et al. reported a stage IIA2 squamous cell carcinoma of a bicornuate bicollis uterus (class IV Müllerian duct anomaly) treated with bilateral intracavitary BT [21]. For fraction 1, the intrauterine catheter was inserted into the right uterine canal, and a radio-opaque marker was inserted into the left uterine canal. The right Manchester A point was calculated as tilted, with the angulated intrauterine source train as well as tilted with the angulated radio-opaque marker in the contralateral uterine canal. This process was repeated for the opposite canals for the second fraction. Three dimensional CT-based planning was performed in four institutions [22,23,24,25]. Platta et al. reported a stage IIB adenocarcinoma of a septate uterus (class V Müllerian duct anomaly), treated with Rotte-Y applicators and 2 ovoid applicators [22]. Five fractions of 5.5 Gy each were delivered to Point A with CT-based treatment planning. Cordoba et al. reported stage IIIA adenocarcinoma of a case of uterus didelphys (class III Müllerian duct anomaly). A total of 20 Gy were delivered to the high-risk clinical target volume (HR-CTV [26]) with BT, using the mold and pulsed-dose-rate techniques [23]. Ishibashi et al. reported the dose-volume histogram comparisons with tandem insertion into the right versus left uterine canals. The comparisons showed wide variations in the projected dose to the CTV and organ at risk. Tandem positioning was chosen to optimize the dose-volume distribution [25].
The standard treatment of locally advanced uterine cervical cancer is CCRT [27,28]. In our institution, 5-6 courses of CDDP 30 mg/m2 has been used mainly for CCRT [29]. Retrospective studies have also reported the use of CDDP 30 mg/m2 [30,31]. In Japan, the standard radiotherapy schedule for stage IIB (large tumor) is as follows: EBRT is given to the whole pelvis; a central shield is used after 30-40 Gy and at the same time, ICBT is commenced. The BT dose for point A corresponding to each central shielding doses are 24 Gy/4-18 Gy/3 fractions [27]. The total doses for point A corresponding to these prescription doses are 62-64 GyEQD2. Furthermore, when rectal or bladder doses are regarded as being equivalent to point A, they are 72.4-73.2 GyEQD2. Since the present case involved double cancers of uterus didelphys, standard therapy could not be applied; therefore, BT dose was increased once more than the standard therapy.
There is no standard RT for carcinoma of the cervix in a uterus didelphys, and the RT for carcinoma of the cervix in a uterus didelphys cannot simply be compared with the treatment for uterus simplex. In the present case, the total doses for point A, the rectum by the ICRU 38, the rectum by calculation and by dosimeter measurement, and the bladder by the ICRU 38 were 72.7, 107.9, 74.1, 64.0, and 107.6 GyEQD2, respectively. Total doses for point A, the rectum and bladder by ICRU 38 were high except for the rectum by dosimeter measurement, compared with the standard dose in Japan [27]. However, the dose was comparable to the values reported in literature, except for low-dose-rate cases [20,22,24,25]. Since the rectal doses by the ICRU 38 and by calculation were relatively high, the grade1 rectal late effect may have developed.
In our case, we discussed the treatment method. Two tandem applicators could not be inserted simultaneously in two uterine cavities with vaginal septum at ICBT, because of the anatomical position and the narrow cervical canal of each small uterus. Therefore, we used one tandem and two ovoid applicators in various ICBT combinations for four times. Only point A was used on the one side of the uterus as the reference point for all 4 sessions of BT; the left side of the uterus was used for three sessions, while the right side of the uterus was used for one session. Tandem insertion was performed three times in the large tumor of the left uterus, the ovoid applicators were put in the left vagina during the first insertion, and bilateral vagina in the second to third insertions, but the dose distribution was focused on the left uterus. In the last (fourth) insertion, a tandem was inserted to the right side to improve the dose distribution in the right uterine body to the uterine fundus. The two uteri were small, and their sounding lengths were 3.5 cm at BT. In Figure 4, which shows the uteri with reference to MRI immediately after the second ICBT, the dose at the right uterine fundus is somewhat lower compared with the left side. However, the tumor contour created with reference to MRI immediately after the second ICBT was surrounded by the distribution of 6 Gy in each of the four ICBT. Thus, the dose distribution is considered to be good for the tumor control on uterus didelphys, because there was no recurrence, even though grade 1 radiation proctitis occurred. The fact that the two uteri were small would likely be one of the reasons why the uterine body to the uterine fundus received a sufficient dose distribution.
At that time, only 2D treatment planning was used in our hospital. In Japan, a nationwide survey was performed in 2012, and 84% (113/135) of BT treatment institutions reported that they used X-ray films for treatment planning [32]. On the other hand, a recent national survey of intracavitary BT in Japan in 2016 showed that 40% (46/115) of BT treatment institutions used X-ray films for treatment planning and in 60% (69/115) of centers, CT images were acquired after applicator insertion. Three-dimensional planning was performed in 48% (55/115) [33].
In future, the use of IGBT would allow for the examination of the optimal dose distribution for the tumor and reduction of the dose to the rectum and bladder. That is, for such a special case, by examining the dose distribution for every intracavitary irradiation, it is possible to discuss how the applicator can be inserted at the next intracavitary irradiation to prescribe the optimal dose for HR-CTV.
Conclusions
The diagnosis and treatment of cancer of uterus didelphys with vaginal didelphys are very difficult. A careful medical interview and pelvic examination of the patient are required at the first visit. In the present case, a combination of EBRT and bilateral uterus BT using 2D treatment planning with concurrent chemotherapy was effective, with no evidence of recurrence or major late complications of the bladder and rectum 80 months after the start of treatment.