Purpose
Uveal melanoma is a rare malignancy, with an incidence of approximately 1,500 cases per year in the United States [1]. Standard of care for uveal melanoma depends on tumor size [2] (Table 1). Treatment modalities that have been utilized for uveal melanoma include observation for certain small tumors, eye plaque brachytherapy (EPBT), proton therapy, stereotactic radio-surgery (SRS), globe preserving surgeries, such as local tumor destruction, and non-globe preserving surgeries (i.e., enucleation) [3]. Based on a prospective randomized controlled trial, EPBT became the standard of care for small size tumors that require treatment and for medium size tumors, and because it is globe-preserving, it shows comparable survival rates compared to enucleation, has a low rate of local failure, and can be successfully salvaged with enucleation [4].
Table 1
Size definitions based on COMS classification
| COMS categories | ||
|---|---|---|
| Basal diameter | Apical height | |
| Small | ≤ 16 mm | < 2.5 mm |
| Medium | ≤ 16 mm | 2.5-10 mm |
| Large | > 16 mm | > 10 mm (or > 8 mm if < 2 mm from optic disc) |
Historically, enucleation was the preferred treatment for large tumors because the available eye-plaques were not large enough to adequately cover these lesions. However, due to the introduction of custom eye-plaques, utilization of ultrasound for intra-operative optimization of plaque positioning and improvement in treatment planning software are now feasible to treat certain large tumors with EPBT [5, 6]. The most recent American Brachytherapy Society (ABS) guidelines stipulate that EPBT may be used for large uveal melanoma, if basal diameters do not exceed the limits of brachytherapy, and there is ≤ 5 mm extra-ocular extension [7]. These guidelines are supported by a handful of contemporary studies, which have reported acceptable outcomes with EPBT in large uveal melanoma [8-12].
Although prospective randomized evidence supports EPBT as the procedure of choice for small/medium tumors, and multiple studies demonstrate feasibility of EPBT in certain large tumors, it is unclear whether practice patterns reflect this. Because it is a rare malignancy, only facilities with adequate patient volume have the incentive to acquire the expertise and resources to implement a successful EPBT program. Analysis of SEER database shows that in the post-COMS era, there has been an overall increase in the percentage of patients receiving radiation and a decrease in the percentage of patients receiving surgery [13, 14]. However, the SEER database studies do not specify the radiation treatment modality employed, and they do not differentiate between enucleation and globe-preserving surgeries, such as local tumor destruction. The purpose of this publication was to look at contemporary trends in the management of uveal melanoma to determine whether practices are appropriately adopting EPBT, and to investigate what demographic and socio-economic factors might be associated with deviations from this standard of care.
Material and methods
Data source and study population
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons (ACoS) and the American Cancer Society. The NCDB collects data from more than 1,500 hospitals with ACoS-accredited cancer programs, accounting for 70% of all newly diagnosed cancers cases in the United States [15-17]. It includes data on tumor characteristics, patient demographics, and survival. Thresholds for income and education were based on NCDB categorization. Thresholds for age and distance traveled were selected by rounding median value. NCDB is not a population-based registry, and under-represents rural areas and minority populations [18]. All pertinent cases are reported regularly from CoC-accredited centers and compiled into a unified dataset, which is then validated. Data used in the study were derived from a de-identified participant user file (PUF) [19]. It was therefore exempted from institutional review board oversight.
Patients’ selection
The NCDB database was queried (2004-2015) for all patients aged 18 or older, with a histologically confirmed diagnosis of uveal melanoma. Patients with nodal disease or distant metastasis at diagnosis were excluded from the analysis. Patients were categorized based on their first treatment modality. Therefore, patients who received a globe preserving treatment followed by enucleation were categorized in the former cohort. Due to missing stage data in some patients and changes in staging from American Joint Committee on Cancer (AJCC) 6th edition to AJCC 7th edition, tumor dimension information and available stage data were utilized to categorize tumors as either small/medium (≤ 10 mm apical height and ≤ 16 mm basal diameter) or large (> 10 mm apical height or > 16 mm basal diameter). Due to stage migration and missing data, it was not feasible to accurately separate small lesions from medium lesions.
Data analysis
Two-sided Pearson χ2 test was used to compare categorical frequencies between patients who received globe preserving treatments vs. those who received enucleation. Line graphs were produced to visualize trends in epidemiology and management of uveal melanoma. Multivariable logistic regression modeling was used to determine characteristics predictive for receiving enucleation. Statistical analysis was conducted using RStudio version 1.2.1335 (R foundation for statistical computing, 2019).
Results
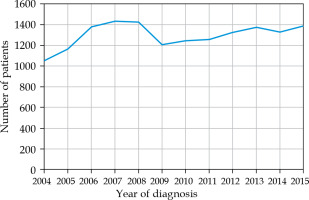
Of the 15,925 patients with histologically confirmed uveal melanoma, a total of 15,662 patients (98.3%) did not have metastatic disease at diagnosis, and thus met the inclusion criteria. Overall, the mean age was 63 years. The mean age was 64 years in the globe preserving treatment cohort, and 62 years in the enucleation cohort. Overall, the number of patients diagnosed with uveal melanoma in participating CoC facilities throughout 2004-2015 averaged 1,327 (range, 1,082-1,459) cases per year (Fig. 1). A total of 9,930 tumors (63%) were classified as small/medium size, 3,336 tumors (21%) were classified as large size, and no size could be determined for the remaining tumors (15%).
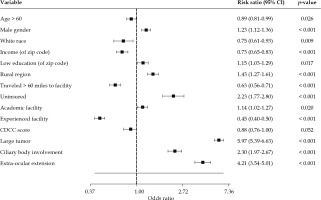
Table 2 displays clinico-pathologic characteristics of the study population. A total of 12,495 (80%) patients received globe preserving treatment, while 3,167 (20%) patients received enucleation. Compared to patients who received globe preserving treatments, patients who received enucleation were more likely to be males, ≤ 60 years old, and non-white race. Socio-economically, these patients were more likely to live in a zip code with a lower median income, live in a zip code with a lower education level, live in a rural region, travel < 60 miles to the treating facility, and not have insurance. Additionally, these patients were more likely to be treated in a non-academic site and be treated in an inexperienced facility (defined as a facility that treated less than median number of patients relative to all facilities overall). Clinically, these patients were more likely to have a lower Charlson-Deyo combined comorbidity (CDCC) score, have large tumors, have ciliary body involvement, and have extra-ocular extension. The overall trend from 2004 to 2015 was 5% less patients receiving enucleation, and 5% more patients receiving EPBT (Fig. 2A). The percent of patients receiving proton therapy or local tumor destruction remained largely unchanged (Fig. 2A). In the small/medium tumor cohort, the enucleation rate decreased from 20% in 2004 to 10% in 2015 (Fig. 2B). In the large tumor cohort, the EPBT rate increased from 30% in 2004 to 45% in 2015, while the enucleation rate remained largely unchanged (Fig. 2C).
Fig. 2
Primary treatment modality by year of diagnosis. A) Overall; B) Small and medium size tumors; C) Large size tumors
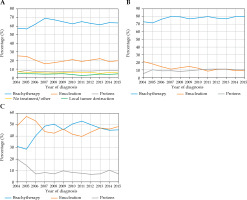
Table 2
Comparison of demographic data of all patients
On multivariable logistic regression analysis, demographic factors independently predictive for receiving enucleation included male gender, age ≤ 60 years old, and non-white race (Fig. 3). Socio-economic factors independently predictive for receiving enucleation included residence in a zip code with lower median income, residence in a zip code with lower education level, rural residence, traveled < 60 miles to the treating facility, and not having insurance (Fig. 3). Facility-related factors independently predictive for receiving enucleation included treatment at a non-academic site and treatment in an inexperienced facility (Fig. 3). Clinical factors independently predictive for receiving enucleation included large tumor, ciliary body involvement, and extra-ocular extension (Fig. 3).
Discussion
In the largest study to date evaluating contemporary treatment trends for uveal melanoma, our results show that the upfront enucleation rate for small/medium tumors decreased from 20% in 2004 to 10% in 2015. This decrease in enucleation rate suggests expanding implementation of EPBT as the accepted standard of care for tumors of these dimensions. For large tumors, our results showed an increased EPBT rate from 30% in 2004 to 45% in 2015, suggesting that with the development of modern eye-plaques providers, the increased comfort in offering this therapy for lesions that historically could not be treated with EPBT.
Overall, the number of patients diagnosed with uveal melanoma in participating CoC facilities has increased to a similar rate of US population throughout 2004-2015. In general, brachytherapy is becoming more prominent relative to surgery because there is increasing evidence for its’ role, growing emphasis on providing adequate brachytherapy training to radiation oncology trainees, and increasing communication between surgeons and radiation oncologists [20]. Furthermore, recent evidence has demonstrated poor overall survival (OS) with proton therapy compared to EPBT, and this may explain why proton therapy utilization decreased from 20% to < 10% for large size tumors in the present study [21].
Our analysis suggests there may be numerous reasons why a small but meaningful number of small/medium tumors are still being treated with enucleation. EPBT is a specialized procedure that requires a skilled ocular oncologist and an experienced radiation oncologist. Because there are only approximately 1,500 cases a year, there are only a limited number of centers with sufficient expertise in this procedure. Centers with this level of expertise are typically academic sites. As expected, our findings suggest that patients who received enucleation were more likely to be treated in an inexperienced facility and non-academic site. We suspect that patients who live in a rural region, in an area with a lower median income, and in a region with a lower educational level were more likely to receive enucleation because they ended up being treated at an inexperienced facility and non-academic site. Likewise, we suspect that those who traveled < 60 miles to the treatment facility were more likely to receive enucleation because they were more likely to have not traveled to a more experienced facility. We speculate that patients of non-white race and without insurance were more likely to receive enucleation because of their lower socio-economic means, and therefore might not have been able to get treatment in a more experienced facility that could offer EPBT. Alternatively, patients of non-white race may be more likely to live in an urban region, which may be a confounding factor. It is unclear why patients ≤ 60 years old and male patients were more likely to receive enucleation. Overall, these socio-economic and regional disparities highlight the importance of initiatives to remove barriers for patients, and to encourage ophthalmologists to refer patients to experienced centers for the management of uveal melanoma.
We suspect that the increased utilization of EPBT for large tumors is due to the advancements in EPBT technology, such as custom plaque designs, intra-operative optimization of plaque positioning using ultrasound, and developments in EPBT treatment planning systems [5, 22, 23]. The COMS trial evaluating EPBT excluded tumors of > 16 mm in basal diameter because the eye-plaques available at that time were not big enough to cover tumors larger than this. A limitation of the current study was that data related to these technological advancements were not recorded in the NCDB, so we were unable to evaluate our suspected association between technological advancements and increased utilization of EPBT for large tumors.
Additional limitations of this study included stage migration from AJCC 6th edition to AJCC 7th edition and the missing data related to variables, including stage, tumor depth, tumor basal diameter, ciliary body involvement, and extra-ocular extension. This limitation prevented us from accurately delineating stage beyond a basic stratification of small/medium tumors and large tumors. Also, the retrospective nature of the study was recognized as a limitation and precluded establishing causal relationships. The NCDB is not a population-based registry and under-represents rural areas and minority populations, because these populations of patients are less likely to be treated in ACoS-accredited cancer programs. Furthermore, the NCDB does not include various additional clinical details, which would be beneficial to the analysis, such as gene expression profile of tumors, local control, and toxicity.
Conclusions
In conclusion, we demonstrated a trend of decreased enucleation for small/medium tumors and increased EPBT for large tumors. A fraction of patients who should be candidates for globe-preserving treatment are instead receiving enucleation, and certain adverse demographic factors are associated with this.