Introduction
Melanoma is one of the most aggressive forms of skin cancer, characterized by rapid metastasis, therapeutic resistance, and poor prognosis in advanced stages [1–5]. Although recent advances in targeted therapies and immunotherapy have improved clinical outcomes, these treatments often come with severe side effects and high recurrence rates [5]. Therefore, the exploration of safer and more effective alternatives remains a pressing need.
Plant-derived compounds have gained increasing attention as potential anticancer agents due to their multi-targeted mechanisms and lower toxicity [5]. Phytochemicals such as phenols, terpenoids, glycosides, and alkaloids have shown chemopreventive and therapeutic properties by enhancing antioxidant activity, activating detoxifying enzymes, suppressing pro-inflammatory signalling, inhibiting angiogenesis and metastasis, and inducing apoptosis [6].
Zanthoxylum acanthopodium DC (ZA), commonly known as andaliman, is a spice widely used in traditional Batak cuisine in Indonesia [7]. The fruit is rich in various bioactive compounds, including alkaloids, flavonoids, saponins, tannins, and triterpenoids [8–11]. Recent studies (2022–2024) have demonstrated that ethyl acetate fractions of ZA fruit exert strong cytotoxic effects against several cancer cell lines, including breast (T47D), colon (HCT-116, WiDr), and cervical cancer cells, mainly by inducing apoptosis through the mitochondrial pathway and modulation of pro-apoptotic genes (e.g., Bax/Bcl-2 ratio) [12–14].
Compared to conventional chemotherapeutic agents such as doxoru- bicin – which are associated with adverse effects like cardiotoxicity and drug resistance – natural compounds from ZA offer selective toxicity toward cancer cells and reduced harm to normal tissues. Furthermore, a recent study suggested that combining ZA extract with doxorubicin could enhance anticancer efficacy while potentially mitigating toxicity [15].
Several bioactive constituents in ZA, such as flavonoids and alkaloids, are known to trigger apoptosis by activating caspase cascades, decreasing the expression of cytochrome c and FasL, and downregulating pro-survival pathways such as PI3K/AKT [11, 16–18]. Given that B16F10 melanoma cells are known for their resistance to apoptosis, targeting these pathways with ZA-derived compounds presents a promising therapeutic strategy.
Therefore, this study aimed to evaluate the cytotoxic and apoptotic effects of the ethyl acetate fraction of ZA fruit on B16F10 melanoma cell cultures. We hypothesized that this fraction induces apoptosis via mitochondrial and caspase-dependent pathways, offering a potential plant-based therapeutic approach for melanoma.
Material and methods
Preparation of ethyl acetate fraction of Zanthoxylum acanthopodium DC fruit
Fresh ZA fruits were collected from the Balige district, North Sumatera, Indonesia. The plant was identified and authenticated by a botanist from the Herbarium Medanese (Universitas Sumatera Utara). The dried fruit was powdered and subjected to maceration in 96% ethanol for 72 hours at room temperature, followed by filtration and evaporation. The crude ethanolic extract was then partitioned sequentially with n-hexane and ethyl acetate to obtain the ethyl acetate fraction (ZA-EAF). The resulting fraction was evaporated to dryness and stored at 4°C [19]. Prior to use, ZA-EAF was dissolved in dimethyl sulfoxide (DMSO) and diluted in the culture medium, with the final DMSO concentration kept below 0.5%. Doxorubicin was used as a positive control for cytotoxic and apoptotic effects due to its well-established activity against melanoma and other cancer cells. It served as a benchmark for comparing the efficacy of ZA-EAF [20].
Reagents
Foetal bovine serum (FBS), penicillin-streptomycin, Dulbecco’s Modified Eagle Medium (DMEM), and 0.25% trypsin-EDTA were obtained from Gibco. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), sodium dodecyl sulphate (SDS), DMSO, and phosphate-buffered saline (PBS) were purchased from Sigma-Aldrich. Hydrogen chloride and ethanol were obtained from Merck. FITC Annexin V (Cat. No. 640945) was purchased from BioLegend.
Cell culture
B16-F10 melanoma cells were cultured in complete DMEM supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells were maintained in a humidified incubator at 37°C with 5% CO2 for 24 hours before treatment.
Cytotoxicity assay
The cytotoxicity of ZA-EAF was evaluated using the MTT assay. Cells were seeded at a density of 5 × 105 cells/ml in a 96-well plate and incubated for 24 hours. Serial concentrations of ZA-EAF (500, 250, 125, 62.5, 31.25, 15.625, and 7.3 µg/ml) were applied in triplicate and cells were incubated for another 24 hours at 37°C. MTT reagent (0.5 mg/ml) was then added and incubated for 4 hours at 37°C. The reaction was stopped by adding 10% SDS in 0.1 NHCl was added, and cells were incubated overnight at room temperature in the dark. Absorbance was measured at 570 nm using an ELISA plate reader. Cells viability was calculated as: viability (%) = 100% × (Abs treated cell – Abs blank)/(Abs control – Abs blank media) [21].
Apoptosis assay
Apoptosis was assessed by flow cytometry using FITC Annexin V and caspase-3 expression analysis. B16F10 cells were seeded at a density of 5 × 105 cells/well in a 6-well plate, and incubated for 24 hours. Cells were then treated with ZA-EAF at concentrations corresponding to IC50, ¾ IC50, and ½ IC50 for 24 hours.
For Annexin V staining, cells were labelled according to the manufacturer’s instruction and analysed by flow cytometry. In parallel, caspase-3 expression was detected using a polyclonal antibody following the manufacturer’s protocol. The percentage of apoptotic cell was quantified and compared across treatment groups.
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Prior to analysis, data were assessed for normality and homogeneity. Due to variance heterogeneity, Welch’s one-way analysis of variance (ANOVA) followed by the Games-Howell post hoc test was used. A p-value of < 0.05 was considered statistically significant. Data analysis was performed using SPSS version 25.
Results
Cytotoxicity activity
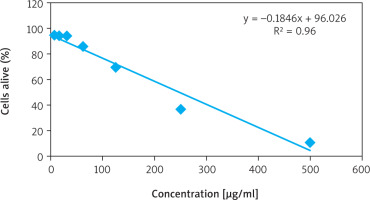
The cytotoxic effect of ethyl acetate fraction of ZA-EAF on B16F10 melanoma cells was evaluated using the MTT assay, which measures cell viability based on metabolic activity. Viable cells reduce MTT to purple formazan crystals through mitochondrial dehydrogenase activity [21].
Treatment with ZA-EAF at concentrations of 500, 250, 125, 62.5, 31.2, 15.6, and 7.8 µg/ml resulted in growth inhibition percentages of 90.2%, 62.6%, 31.3%, 17.3%, 4.9%, 9.0%, and 8.3%, respectively. The IC50 value was determined to be 249.3 µg/ml, with a linear regression coefficient (R) of 0.9603, indicating a strong positive correlation between ZA-EAF concentration and its cytotoxic effect on cell viability (Figure 1) [22]. Based on the IC50 value (249.3 µg/ml), cells were categorized into three treatment groups: IC50 (249.3 µg/ml), ¾ IC50 (187 µg/ml), and ½ IC50 (124.5 µg/ml).
Apoptosis induction
Apoptosis induction was assessed using Annexin V-FITC/PI staining followed by flow cytometry analysis. Zanthoxylum acanthopodium DC EAF treatment increased the proportions of apoptotic cells in a dose-dependent manner, as indicated by the rise in both early (Annexin V+/PI–) and late (Annexin V+/PI–) apoptotic populations (Figure 2).
Figure 2
Flow cytometry analysis of apoptosis induction using Annexin V-FITC/PI staining Quadrants: Q1 (necrotic), Q2 (late apoptotic), Q3 (live), Q4 (early apoptotic)
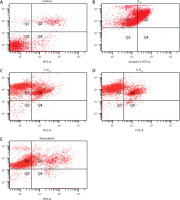
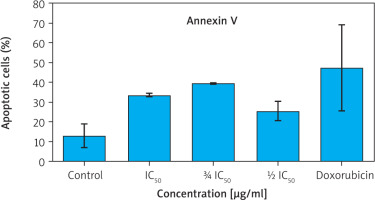
Figure 3
Percentage of apoptotic cells (early and late apoptosis) based on Annexin V staining
Data are presented as mean ± SD. Although intergroup differences were significant, no significant differences were observed when compared to the control group (p > 0.05, Games-Howell post hoc test).
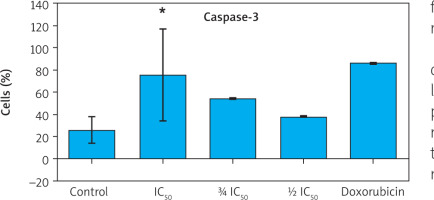
To further confirm apoptosis induction, caspase-3 expression was measured using flow cytometry. Zanthoxylum acanthopodium DC EAF treatment at all tested concentrations resulted in a dose-dependent increase in caspase-3 positive cells compared to the control group (Figure 4).
Figure 4
Flow cytometry plots showing caspase-3 expression in B16F10 melanoma cells after treatment with Zanthoxylum acanthopodium DC ethyl acetate fraction (IC50, ¾ IC50, ½I C50) and doxorubicin
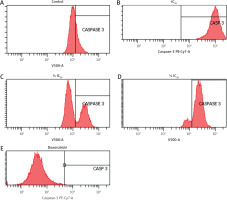
Statistical analysis demonstrated significant differences in caspase-3 expression among treatment groups (p < 0.001, Welch’s ANOVA). Post hoc analysis comparisons confirmed significantly higher caspase-3 expression in all ZA-EAF treated groups compared to the control group (p < 0.05) (Figure 5). These findings confirmed that ZA-EAF activates the caspase-dependent apoptotic pathway in B16F10 melanoma cells in a dose-dependent manner (Figure 5).
Discussion
This study demonstrates that the ethyl acetate fraction of Zanthoxylum acanthopodium DC (ZA-EAF) exhibits dose-dependent cytotoxic activity against B16F10 melanoma cells as confirmed by the MTT assay. Higher concentrations of ZA-EAF significantly reduced cells viability, indicating its potent anticancer potential. This finding aligns with a previous study reporting cytotoxic effects of various ZA extracts in multiple cancer cells lines, including T47D, 4T1, MCF-7, HeLa, and Raji cells [12, 23, 24]. The cytotoxic effect of ZA-EAF is likely attributable to its diverse bioactive constituents such as alkaloids, flavonoids, and steroidal compounds, which are known to modulate cellular homeostasis and induced programmed cell death [8].
Alkaloids, for instance, exert cytotoxic effects by interfering with DNA replication, inhibiting telomerase activity, and destabilizing cytoskeletal proteins, which collectively trigger apoptosis [25]. Flavonoids scavenge reactive oxygen species, disrupt the cell cycle, promote apoptosis, and attenuate tumour invasiveness [26]. Steroidal glycosides, such as cucurbitacins, are capable of inducing mitotic arrest and initiating caspase cascades, and are under investigation as promising therapeutic agents [25]. Interestingly, the IC50 value of ZA-EAF in B16F10 cells (249.3 µg/ml) was substantially higher than in T47D cells breast cancer cells (48.9 ±0.3 µg/ml) [23], suggesting that melanoma cells are more resistant due to higher mutation burden and enhanced proliferation capacity [27, 28]. These differences highlight the need for deeper mechanistic exploration of cell-specific responses to phytochemicals.
Apoptosis plays a critical role in cytotoxic effect of anticancer agents. The current study revealed a significant increase in caspase-3 expression following treatment with ZA-EAF at IC50, indicating the activation of apoptotic pathways in B16F10 melanoma cells. Caspase-3 is a key executioner caspase involved in the intrinsic and extrinsic pathways, and its activation leads to cleavage of structural and regulatory proteins, eventually causing chromatin condensation, membrane blebbing, and apoptotic body formation [29–32].
However, Annexin V staining did not show significant increase in phosphatidylserine (PS) externalization, suggesting a temporal discrepancy in apoptotic progression [33]. Previous studies have indicated that caspase activation can precede detectable PS exposure, particularly in the early and delayed apoptotic phase [30]. This finding emphasizes the importance of conducting time-course analyses and integrating multiple apoptotic markers to better characterize cell death dynamics.
Despite the observed cytotoxic and apoptotic effects, the precise mechanisms by which the ZA-EAF exerts its effect remain to be fully elucidated. Further studies using complementary approaches, such as transcriptomic or proteomic profiling, are warranted to determine the changes in gene expression, signalling pathway modulation (e.g., PI3K/AKT, NF-κB, MAPK), and oxidative stress response. Additionally, the impact of ZA-EAF on mitochondrial membrane potential, intracellular calcium levels, and autophagic flux could provide insight into the multifaceted mechanisms involved.
Cytotoxic and apoptotic effects are inherently interrelated. Many chemotherapeutic agents induce cytotoxicity by activating apoptosis, thereby suppressing tumour growth while minimizing collateral damage to normal cells. Understanding this relationship is crucial for identifying therapeutic windows and optimizing the use of plant-based compounds in cancer treatment.
Future perspectives
The current findings support the potential of ZA-EAF as a source of novel anticancer agents, particularly for drug-resistant melanoma. Future research should focus on identifying the specific active compounds responsible for its bioactivity and clarifying their mechanisms of action. Moreover, in vivo validation and combinatorial studies with standard chemotherapeutics, such as doxorubicin, could help evaluate the efficacy and safety of ZA-EAF in complex biological systems.
Conclusions
This study highlights the cytotoxic and pro-apoptotic potential of the ethyl acetate fraction of ZA-EAF against B16F10 melanoma cells. Zanthoxylum acanthopodium DC EAF demonstrated dose-dependent cytotoxicity and significantly upregulated caspase-3 expression, suggesting activation of apoptosis pathways. These findings support the possibility of ZA-EAF serving as a natural candidate for melanoma therapy, particularly in targeting apoptosis- related mechanisms.
However, to translate these findings into clinical application, further research is required to elucidate the underlying molecular pathways, assess its selectivity and safety profile on normal cells, and validate its efficacy in in vivo models. With continued investigation, ZA-EAF holds potential as a complementary or alternative strategy for melanoma treatment.