Purpose
Brachytherapy is usually associated with treatment of malignant diseases, and the most common example is local radiotherapy of the prostate tumor using iodine-125 (125I) seeds. Lesser-known are possibilities of brachytherapy in the treatment of benign diseases, in which radiotherapy can also refer to decades of positive experience [1, 2]. Examples include prevention of heterotopic ossification after hip arthroplasty and inhibition of disease progression in different hyper-proliferative or inflammatory disorders [3]. Another field of application is prevention of in-stent restenosis by intra-vascular brachytherapy [4], for coronary [5] as well as peripheral arterial occlusion diseases [6]. Radio-biological mechanisms are still not fully understood, but the formation of both inflammatory cytokines and those of certain growth factors, can be reduced by ionizing radiation with doses in the range of 10 to 20 Gy [7, 8]. Since in benign diseases unnecessary radiation exposure of healthy tissue is even less tolerated than in cases of tumor irradiation, brachytherapy with low irradiation depth, and thus reduced damage to surrounding tissue of target volume, is at an advantage over teletherapy. This makes 32P a radioisotope of choice for applications, where radioactive source is in intimate contact with targeted tissue. 32P emits electrons of 690 keV mean energy (1.7 MeV maximal energy), leading to a maximum range of about 6 mm in tissue.
A particularly suitable field of 32P application for low-dose-rate (LDR) brachytherapy could be prevention of restenosis in hollow organs, if after surgical intervention excessive scarring during wound healing occurs requiring further medical intervention. Wound healing begins with an inflammatory phase, which dominates in the first two days after injury, followed by a proliferation phase in the following days. Both processes can be influenced by ionizing irradiation, and 32P with a 14.3 day half-life fits well into this time scale of wound healing. Successful treatment of hyper-proliferative disorders with specially developed 32P implants has been demonstrated in pre-clinical studies of glaucoma filtering surgery [9] or prevention of restenosis of para-nasal neo-ostia [10]. However, there are only a limited number of approved radioactive implants available, especially for LDR brachytherapy. This restricts the possibilities for brachytherapy applications.
This report presents development of a new kind of radioactive 32P-foil, which is intended for adaption to different application geometries, but for use in hollow organs in particular. The feasibility of this concept was tested in a dedicated pre-clinical in vivo model setting, associated with prevention of benign urethral stenosis. Primary radiation effects were observed. However, the study duration excluded further radiobiological investigations of stenosis development.
Material and methods
The radioisotope 32P is usually generated by activation of stable phosphorus-31 (31P) via the neutron capture reaction 31P(n, γ)32P, which has a capture cross-section of only 170 mbarn for thermal neutrons. The activation is therefore best carried out at a high-flux neutron source. 31P activation can be performed before or after incorporation into a therapeutic applicator. The former possibility, originally developed for 32P coronary stent fabrication by ion implantation [11, 12], has been already applied in pre-clinical studies for neo-ostium stents [10], and even for bio-resorbable implants in glaucoma therapy [9]. Although ion implantation has been shown to be quite flexible, this technique was considered to be too complex and costly. Therefore, an easier production process was chosen, starting with 31P containing foils, which can be prepared and stored for later activation on demand.
Development of 31P-containing foils and prototype production
Due to the neutron activation process, the foil polymer must withstand a neutron and gamma dose of many MGy without significant changes in material properties. Extensive irradiation tests on several bio-compatible polymers showed that polyetheretherketone (PEEK) meets these requirements best [13]. This polymer is bio-compatible, chemically highly resistant, and applicable at temperatures up to at least 250°C. PEEK melts compared to most other thermo-plastics at a relatively high temperature, close to 341°C. In the range of its’ melting temperature, it can be processed using cost-effective extrusion methods of granulate mixed with a 31P-containing additive. A suited additive must be stable up to the PEEK melting temperature, and must not contain components forming long-lived radioactive nuclei during neutron activation. Sodium metaphosphate (NaPO3) was selected as phosphoric compound because of its’ non-toxic quality, available in powder form, and thermally stable up to 550°C. Moreover, short-lived gamma emitter 24Na (T1/2 = 15 h) only is created during activation, which can even serve as an additional monitor to cross-check the 32P activity. Foil production started by mixing of PEEK granules (PEEK 381G natural, Victrex, England) with 25 wt% NaPO3 powder in a screw compounder and subsequent melt filtration to obtain a fine-particle compound granulate. From this material, a 50 μm thick foil was extruded, which revealed a homogeneous NaPO3 distribution in first transmitted light inspection. Due to the use of experimental production plant, the variation of NaPO3 content was to be expected at about 15%; therefore, the exact final 32P activity of an individual foil implant had to be determined later.
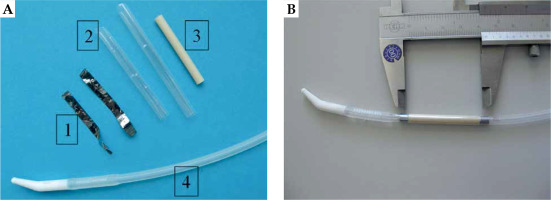
Because an indwelling catheter is typically inserted in the urethra after surgical intervention, the novel approach of this study was to use this urethral catheter as a carrier of the activated 31P-foil. An advantage of this approach is that the later clinical application procedure of the catheter can remain unchanged, except for necessary radiation protection measures. Particular attention was drawn to a sufficient foil length, such that the stenosis area could be irradiated with the desired dose, even with possible positioning errors and displacements of the implant, and thus avoid the so-called ‘edge effect’ known from coronary 32P-stents [14]. The observed stent end stenosis was traced back to the dose drop at the stent ends, where still injured areas would heal in the presence of low radiation levels, which can even stimulate proliferation. Hence, rectangular pieces were cut from the 31P-foil stock of 4 cm length and about 3 cm width fitting to form a double-layer around the catheter. These two foil layers can reduce dose inhomogeneities due to production-related unequal phosphor grain distribution, and also halve the neutron activation time. The roll reached thermoplastic form stability by tempering at 220°C for two hours. Additionally, a 10 mm diameter circular plate was punched out from the same foil stock area, which served as monitor for quality assurance. Foil rolls and monitor plates were weighed and further processed only those, which were within a 5% area weight window, i.e., with corresponding NaPO3 content. Due to the production process, NaPO3 is distributed within the foil up to the surface, and could potentially be dissolved and washed out during later application. Therefore, before activation, all foil rolls were thoroughly washed in a shaker for two hours in a mixture of 90% VE water and 10% purest ethanol. Selected pieces were welded under argon atmosphere in dedicated activation capsules. After foil activation and appropriate decay time for 24Na, a 10 mm thick plexiglass shielding was used for radiation protection in final assembly of the urethral catheter (indwelling catheter, Rüsch-Care, Teleflex Medical GmbH, Germany). The 32P-foil roll was slipped onto the catheter tube, and two Ta X-ray markers were glued to both foil ends (Figure 1). Various sealing concepts were tested to prevent subsequent 32P wash-out. This involved either one or two overlapping bio-compatible shrink tubing (Polyester, Advanced Polymers, Salem NH, USA). Alternatively, one shrink tube with circular end sealing was applied using different adhesives, including Loctite 4061 (Medical Line, Loctite, Garching, Germany), Technomelt (Henkel, Munich, Germany), and Dymax 222/100 (Dymax Europe, Frankfurt, Germany). Before application in animal tests, the whole foil-catheter setup was sterilized by 30 kGy 60Co gamma radiation.
Activity and dose distribution determination
Neutron activation was performed using a capsule irradiation system of research neutron source (FRM II) at the Technical University of Munich (TUM, Garching, Germany), which offers a thermal neutron flux density up to 1014 cm-2 s-1. Due to the position-dependent neutron flux, each capsule contained an additional Au standard for determination of a particular integrated neutron dose by gamma spectroscopy. Following neutron activation of 20 minute typical duration, implant prototypes were first subjected to measurements of 32P activity including wash-out tests and determination of dose distribution, which required special effort due to low electron range of only a few millimeters. Dose measurements were performed according to existing protocols of the AAPM TG-43 and TG-60 for interstitial brachytherapy sources with low-energy gamma radiation [15, 16]. For direct determination of the 32P activity, a liquid scintillation counter (LSC) was used (Tri-Carb 2900 TR with scintillator Ultima Gold AB, Perkin Elmer, Germany). This method, however, is calibrated only for liquid radioactive materials, whereas PEEK has a very poor solubility. Therefore, the unknown detection efficiency for activated monitor plates was calibrated via gamma spectroscopy of the stoichiometric component 24Na, which decays almost exclusively by emission of high energetic gamma radiation (1368.6 keV, 2754.0 keV). Potential 32P wash-out of the radioactive catheter setup was also determined by means of LSC.
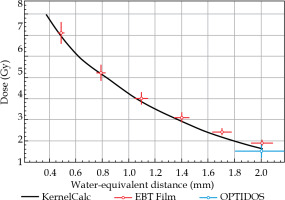
Dose distributions of 32P-foil rolls were both calculated and measured for water environment as proposed in Ref. [17], in view of the later close contact to urethral tissue. MC simulation toolkit Geant4 [18] was used to model beta decay of 32P and related electron dose deposition, including Bremsstrahlung, which tracks electrons down to an energy threshold of 150 eV. An example of a Geant4 dose simulation of a 14 Fr catheter equipped with a 32P-foil roll of 1 cm length and 100 kBq activity is shown in Figure 2 (left). It demonstrates the short range of emitted beta radiation and therewith simple radiation protection by 10 mm thick plexiglass. Additionally, a much faster dose-point kernel calculation was developed (KernelCalc) using a superposition of pre-calculated, equally distributed point-like 32P sources, for which an example is given in Figure 2 (right) for comparison to Geant4. Dose distributions calculated with both methods were found to be in good agreement, as shown in Figure 3, where the radial Geant4 dose distribution was compared to that of the dose kernel calculation. The intended 15 Gy tissue dose at 1 mm distance from the foil surface can be reached within 7 days by 264 kBq start activity only. It should be noted that this cumulated 15 Gy dose results in a tissue contact dose of about 80 Gy.
Fig. 2
7-day dose distribution of a 32P-foil roll in water with 1 cm foil length, 14 Fr diameter, and 100 kBq start activity; simulations by Geant4 (A) and KernelCalc (B) are shown in a plane through the roll axis
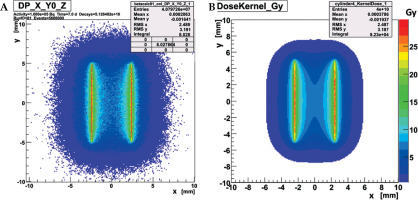
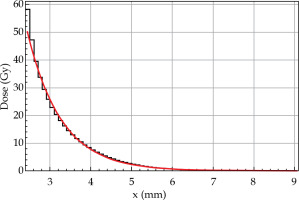
Fig. 3
Radial dose distribution of 32P-foil roll shown in Figure 2 at y = 0 mm: simulated doses by Geant4 (black) and KernelCalc (red) are from the foil surface outwards
For experimental verification of the calculated dose distributions, measuring techniques with about 1 mm spatial resolution are required. OPTIDOS system (PTW, Freiburg, Germany) was developed especially for brachytherapy sources, such as prostate seeds, and fulfills this requirement. The actual detector consists of a cylindrical scintillation crystal with 1 mm diameter and 1 mm length, thus achieving the required spatial resolution in the range of 1 mm and absolute accuracy of 16%. Dose distribution for source distances below 2 mm was measured with a radio-chromic film stack (Gafchromic EBT3, Ashland Advanced Materials, USA), whose 0.3 mm single-film thickness provided adequate depth resolution. The EBT3 film was calibrated with 6 MV photons, using the known dose equivalence to electrons within an error of 6% [19].
Animal model and preparatory experiments
Male rabbits (New Zealand line, aged 6-7 months, weight: 3.5-4 kg) were selected as in vivo model because their urethra can be treated with existing instruments from pediatric urology. Three treatment groups (each, 6 animals) were enrolled, with a 30 Gy or 15 Gy cumulated 7-day dose within 1 mm urethra tissue depth and 0 Gy (control). The targeted dose with the 32P implant was based on the studies for prevention of coronary in-stent restenosis with 32P stents [20], since the urethra of rabbits has comparable dimensions. The following study protocol was developed for a small cohort in vivo feasibility study, with specific regard to proof-of-concept application on urethral stricture guided by clinical procedure: induction of a urethral stricture (day 0); stricture check and surgical opening by urethrotomy (according to Sachse), catheter insertion (day 28); catheter removal after 30, 15, or 0 Gy cumulated dose (day 35); rabbit sacrifice, urethra removal and preparation for subsequent histological analysis that included different staining procedures (e.g., H&E, EvG) (day 63). At each step, the urethra was characterized by an optical and a radiological method (video-urethroscopy and cysto-urethrography). This study protocol is in accordance with the German Animal Welfare Act, and approved by local authorities of Upper Bavaria (approval No., 55.2-1-54-2531-69-08).
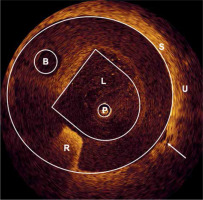
Two preparatory experiments were performed to test new methods for positioning control of the urethral catheter on the one hand, and for induction of a urethral stenosis on the other hand. Due to a steep dose drop of beta radiation, close contact to the urethral tissue and of the 32P-foil was essential, which depended mainly on a proper size of the catheter. This tissue-foil contact was checked with optical coherence tomography (OCT) on two rabbits. Endoluminal radial OCT investigation was performed using a time-domain OCT (Type M2x, LightLab Imaging Inc., UK), with a light of a super-luminescence diode (1300 nm, 25 mW) [21]. After insertion of a 14 Fr catheter into the bulbar urethra, the radial OCT probe was introduced into central passageway lumen of the catheter. Within the relevant range, the tissue was in close contact with the implant, besides a few places of a few millimeters in length, where a gap of a maximum of 0.4 mm occurred (Figure 4).
Fig. 4
OCT-cross section image of a 14 Fr catheter inside a rabbit urethra: (P) Radial OCT probe, (L) Passageway lumen, (B) Balloon dilation channel, (R) Reflex of radiopaque material, (S) Outer catheter surface, (U) Urethral tissue. The arrow points to a 0.4 mm gap between the catheter and the tissue
Urethral strictures were induced by a technique developed originally for endovenous laser ablation of varicose veins. Laser light (1470 nm diode laser) was endoscopically introduced with a radially emitting laser fiber [22]. Precise positioning of the fiber and reproducible radial irradiation were enabled using a circular pilot light under optical control. Application of about 100 J light energy (10 W, 10 sec) was found sufficient for successful circumferential thermal destruction of the urethral tissue.
Results
Measurements on activated prototypes
Simulated and calculated dose distributions were first checked on monitor plates, where both dose and activity could be measured easily and extrapolated by simulation to the corresponding foil rolls. A dose distribution, measured by an EBT3 film stack, and corrected for water-equivalent thickness, is presented in Figure 5 to a KernelCalc calculation using LSC determined plate activity as input. Additionally, in Figure 5 at a 2 mm distance, the dose value measured with the calibrated OPTIDOS system is plotted confirming the overall good agreement of different methods within estimated experimental errors of 6% (EBT) and 16% (OPTIDOS), respectively. The lateral EBT3 resolution also offered a possibility to control the 32P grain distribution, which was found to be sufficiently homogeneous (without ‘hotspots’), and was additionally smoothed by two foil layers. Measuring the 24Na activity by gamma spectroscopy and 32P activity with LSC of monitor plates, a linear relation was found between activity and weight. Consequently, precision of the intended implant activity could be adjusted to 5% by selecting foils of a 5% surface weight range.
Fig. 5
Dose distribution of a 32P monitor foil plate in water (measurement with EBT film stack and OPTIDOS) compared to KernelCalc simulation for 35.8 kBq activity (as measured with LSC)
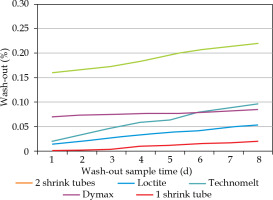
32P wash-out tests of activated catheter assemblies with various sealing techniques yielded by far the best result for two overlapping shrink tubing. A cumulative wash-out within 8 days of only 0.02% of the start activity was measured (Figure 6). A maximum of 200 Bq activity wash-out within 4 hours is allowed for a sealed radioactive source, according to German standard DIN 25426-4 [23], which can be easily fulfilled by a two-tubing sealing for at least 1 MBq start activity, four times more than the envisaged implant activity. Apparently, the adhesives could not be securely enough attached at single shrink tube ends to prevent ingress of liquids by capillary effect and successive wash-out. Therefore, 32P foils were finally sealed by means of two overlapping heat shrink tubes of 25 μm wall thickness, as shown in Figure 1.
Application test of urological 32P catheter prototype on rabbits
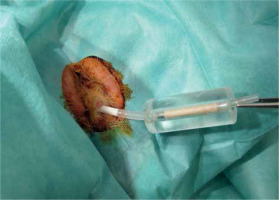
First, according to the study protocol, a defined stricture had to be generated in the rabbit urethra located about 15 mm distal to the colliculus seminalis. After application of 100 J of laser light energy, a pronounced induced stricture developed during the following 28 days, which was characterized both by cysto-urethrography and video-urethroscopy. According to the stricture classification, as shown in Figure 7, different stricture grades were observed, including grade 3 in 15 of 18 animals (83%), grade 2 in 1 of 18 animals (6%), and grade 1 in 2 of 18 animals (11%). To mimic the clinical treatment procedure, strictures were surgically slitted lengthwise at 12 o’clock, until the full width of lumen was reached. Immediately after surgical opening of the urethra, gamma-sterilized 14 Fr catheter carrying the specially developed 32P-foil roll was inserted. The foil position was matched with the stricture by radiography using two X-ray markers, and the catheter balloon was filled with contrast agent and blocked in the bladder neck. During the whole procedure, beta radiation was shielded by a 10 mm thick plexiglass cylinder covering the 32P-foil area (Figure 8). All individuals working with the radioactive catheter were equipped with ring batches and an extremity dosimeter.
Fig. 7
Stenosis grade definition by cysto-urethrography (X-ray, right images) and video-urethroscopy (left images)
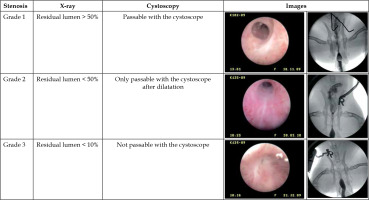
Fig. 8
Insertion of a 32P-foil catheter prototype inside a plexiglass radiation protector into a male rabbit urethra
On day 35 after stenosis induction (7 days after surgical treatment with catheter introduction and start of irradiation), LDR brachytherapy was finished by removal of the catheter. Four weeks later, on day 63, the animals were sacrificed and their urethra was prepared for later histopathological analysis focusing on early radiation-induced effects. Urothelium inflammation was identified in tissue slides by influx of pseudo-eosinophile granulocytes, which are a specific indicator for rabbits. An averaged inflammation grade by normalized influx of 0.83 for 0 Gy, 0.33 for 15 Gy, and 1.17 for 30 Gy was determined, but without statistical significance. Mild radiation damage characterized by specific changes of medium and small vessels was observed for both dose groups. Although significant LDR-related biological effects could not be stated due to the short observation time and small number of animals, the intended feasibility and practicability of this treatment procedure was demonstrated successfully.
Discussion
Stenosis in hollow organs is a common clinical problem caused by a variety of reasons, often of iatrogenic traumatic or inflammatory etiology [24]. Examples in urology are transurethral interventions or catheter inserts, including anastomosis strictures after surgical interventions, such as prostatectomy, with an incidence rate of 1.1% to 8.4% [25]. As a first therapy option, stenoses are usually dilated or slit with temporary catheterization. However, wound healing following this procedure often leads to stenosis recurrence, and further operative revisions commonly end up again with the same problem [26]. In this unsatisfactory situation, the proposed LDR brachytherapy approach could be a possible alternative for stenosis or restenosis prevention.
For this reason, several studies based on brachytherapy have been performed using both commercially available systems and specific in-house radiation sources. For comparison with the 32P-foil system described here, some typical irradiation devices are mentioned as examples, which have already been used in clinical as well as pre-clinical studies on stenosis prophylaxis. In urology, after trans-urethral slitting of stenosis in 15 patients, a dose of 4 Gy within a 3 mm tissue depth was applied directly on the following two or three days after surgery by inserting a commercially available iridium-192 (192Ir) afterloading line source through the lumen of lying catheter [27]. Another clinical trial with 192Ir-high-dose-rate (HDR) brachytherapy was conducted on 17 patients with different stricture history [28]. Here, a cumulative dose of 10 to 15 Gy was administered within 3 post-operative days. In animal studies, an experimental balloon catheter filled with the beta emitter 188Re (t1/2 = 17 h, Emax = 2.12 MeV) in liquid form was used for HDR brachytherapy [29]. After stenting of a dog urethra, doses of 20 and 40 Gy were delivered at a 1 mm distance from the balloon surface adapting a technique, which has also been used for prevention of in-stent restenosis in coronary arteries. Another approach, somewhat similar to the present investigation, has been chosen in a pre-clinical LDR brachytherapy study on prevention of bile duct stenosis. A self-expanding nitinol stent was covered with a PU film loaded with the beta emitter 166Ho (t1/2 = 26.8 h, Emax = 1.85 MeV) [30]. Preliminary reports on prevention of bile duct as well as urethra stenosis with LDR brachytherapy using 32P foils were previously presented [31, 32].
The present study presents an innovative and technically uncomplicated method, which may prevent surgically treated urethra from restenosis. The decision to rely on a radioactive foil proved to be a suitable choice that fulfilled all requirements of the envisioned application. Foil production is a relatively simple and inexpensive process, and many bio-compatible polymers are available, which can contain different compounds, and with PEEK, even a sufficiently radiation resistant polymer is at hand. In addition, PEEK with its’ high melting temperature enables different sterilization methods, including gamma sterilization. Foils can be produced in large quantities, which can be stored, activated, and applied on demand. Dose distribution along a foil surface is more homogeneous as opposed to nitinol stent, for example. The pre-rolled 50 μm thick foils were easily slid onto the urinary catheter and fixed with shrink tubing; thereby, enabling a minimally altered procedure for catheter implantation in clinics. The observed 32P wash-out may be further reduced by additional coating of the foil surface during production. Notwithstanding the simplicity of this approach, a foil-based implant has limited applicability and is mainly suited for use in hollow organs. The implant production method, however, can be adapted to other applications. A new fabrication technique based on 3D printing (additive manufacturing or fused deposition modeling) of medical devices has become an emerging technology in recent years, which enables a much more flexible, yet personalized design of implants that can open new perspectives for brachytherapy [33, 34]. Meanwhile, even PEEK-based devices have been produced with this technique [35], which could incorporate also a 31P component; therefore, overcoming the geometrical constraint of foils and extending this therapy concept to further clinical challenges.
31P is the only stable phosphorous isotope, such that no other disturbing isotope besides 32P is formed during neutron activation. Selection of the non-toxic, temperature-stable, and crystalline compound NaPO3 was adequate. After activation, it adds only a short-lived isotope 24Na to radioactive inventory, which could even be used for calibration purposes. Considering the short range of 32P beta radiation, special care was taken to obtain the urethral tissue in close contact with the foil surface. Due to associated surgical trauma, an inflammation-related swelling in the stenosis area is to be expected, which additionally increases the tissue-foil contact; thus, ensuring that the desired dose actually reaches targeted volume. In this sense, the proposed implant is self-adjusting. Assuming a homogeneous surrounding of radiation source, calculation of dose distributions could be facilitated by using a fast dose kernel method. Thinkable extensions of this brachytherapy approach, even to malignant diseases, must consider beta radiation specific steep dose drop and connected high contact dose (as shown in Figure 3). For example, irradiation of superficial dermatological tumors [34], such as basalioma or melanoma with a ‘32P-film patch’ could become an option, similar to the dura brachytherapy plaque used in Ref. [36].
It is shown that the urethra of male rabbits is a well-suited animal model for application tests of this new radioactive catheter. As a prerequisite for this study, a reproducible stricture induction technique was essential. Using radial laser light irradiation visually guided by a pilot light, a circumferential stricture could be precisely located on all animals in the urethra. However, for a significant therapeutic outcome relevant to brachytherapy, time schedule of such a study needs to be correlated with induced biological processes. Because the mean acute wound healing processes takes 7-10 days, while full healing is completed after several months, it can be assumed that only a primary radiation effect would be observed during a defined follow-up time. The measured lowest inflammation grade within the 15 Gy group could be interpreted as indication of inflammation suppression at this dose. Interestingly, the overall mild radiation damage for contact dose up to 80 Gy correlates with the observed radiation tolerance of the human urothelium [27]. Restenosis formation occurs mainly during a long healing process period, which was not in the scope of this animal experiment and needs further investigation.
The question of whether LDR or HDR brachytherapy is more suitable for prevention of post-operative stenosis or keloid scar formation and, more generally, whether there are different underlying biological mechanisms of both irradiation modalities, remains open [37]. The presented pre-clinical LDR brachytherapy study using a 32P-foil catheter with its’ high reproducibility, can motivate a comparative study with available 192Ir or 188Re HDR irradiation sources.
Conclusions
This proof-of-concept study describes development and testing of a novel approach to endoluminal LDR brachytherapy for treating restenosis in hollow organs. The concept based on PEEK foils containing the beta emitter 32P is demonstrated to be a practicable and safe procedure during animal test on laser-induced strictures of the rabbit urethra. This implant design, where the radioactive foil is wrapped around the urethral catheter, enables the application of a prescribed dose distribution in a precise and computable manner. A possible extension of this method to other clinical cases with similar restenosis problematics, such as bile duct stenosis, seems promising. Because results of a dose response study in an animal model may differ significantly from human tissue response, further studies are warranted within dedicated clinical investigations.