Purpose
External beam radiation therapy (EBRT) in combination with high-dose-rate (HDR) brachytherapy (BT) is now considered as the definitive treatment strategy of locally advanced cervical cancer. The gold standard in adaptive brachytherapy is image-guided adaptive brachytherapy (IGABT), which enables individualized treatment planning and delivery [1].
Lymph node metastases are common in locally advanced cervical cancer patients. According to previous research of Liu et al., the obturator and external iliac nodes are the most frequently metastasis [2]. Furthermore, RetroEMBRACE cohort studies showed that pelvic failures account for 13% of treatment relapse following cervical cancer chemoradiation therapy [3]. Therefore, dose to pelvic lymph nodes is crucial to be determined during treatment time and clinical decision-making for any potential future relapse.
Nowadays, among all BT applicators, computed tomography (CT)/magnetic resonance imaging (MRI) compatible tandem-ovoid (TO) and tandem-ring (TR) intra-cavitary applicators are the most commonly used to treat locally advanced cervical cancers, with or without gross target volume (GTV) residue after EBRT. With a TR applicator, insertion is more comfortable, and reproducibility is better for patients with a narrow vagina or obliterated fornix [4]. Some studies investigated the difference in resulting dose distribution using these two applicator types [5, 6]. Indeed, the dose to organs at risk (OARs) during BT procedure differs according to applicator set: TO or TR ICRU-related point dose and [7]. While radiation oncologist’s manually delineated contours are considered as the gold standard in current clinical practice, manual delineation of all regions of interest (ROIs), such as pelvic lymphatic chains, is time-consuming, labor-intensive, and difficult in radiation therapy planning [8]. Historically, the absorbed dose at Manchester point B was used to estimate the cumulative absorbed dose to the internal iliac and obturator lymph nodes as well as the pelvic sidewall from BT and EBRT. In addition, recent Siavashpour et al. study on HDR-BT of cervical cancer with 60Co source, discovered certain correlation between the external iliac hot points and point B [9].
These findings demonstrate that it is clinically prudent to know the dose to the pelvic lymph nodes and their relationship with easily definable Manchester point B dose during cervical cancer BT, using any clinically familiar source and applicator set. Therefore, the purpose of this study was to determine the correlation between dose delivered to the pelvic lymph nodes and point B using TR applicators for intra-cavitary brachytherapy treatment of locally advanced cervical cancer.
Material and methods
The current study was a retrospective study conducted at Lucien Neuwirth Cancer Center (Saint-Priest-en-Jarez), France. The study, which was carried out in accordance with the Helsinki Declaration, was authorized by the institutional ethics committee. Patients medical data were used in this retrospective study, and oral and written informed consent was obtained.
Case selection and pre-planning
Cervical cancer patients treated by intensity-modulated radiotherapy (IMRT) or 3D conformal radiotherapy alone to a total dose of 45-50.4 Gy with concurrent weekly cisplatin-based chemotherapy at Lucien Neuwirth Cancer Center, from 2015 to 2018, were included in this study. Additional boost phase to the involved lymph node was prescribed to rich a cumulative dose of 50.4 Gy. Once EBRT was completed, T2- and T1-weighted MRI were obtained before first BT fraction, to determine any gross residual tumor without applicator in situ. Only patients without any interstitial BT component were considered for inclusion into the study.
Patient preparation and applicator insertion
Each patient had a soft diet for 24 hours, after which fasted for 12 hours before applicator insertion. TR brachytherapy applicator insertion was performed under epidural sedation in the operation room before first brachytherapy session. Bladder Foley catheter, with a contrast-filled balloon (1 cm3 contrast plus 6 cm3 normal saline) and a rectal retractor were also inserted. Tandem ring-applicator sets were applied (Varian Medical Systems, Palo Alto, CA, USA). Plastic caps of ring applicator were in place to avoid excessive dose to vagina mucosa.
Imaging, contouring, and treatment planning
A pelvic CT scan with a slice thickness of 2 mm was performed. At EBRT completion, a pelvic MRI was used to achieve BT target delineation. Images were imported to treatment planning system (Eclipse; Varian Medical Systems, Palo Alto, CA, USA). Applicator reconstruction, high-risk clinical target volume (CTVHR), and low-risk clinical target volume (CTVIR) delineation were performed in compliance with the Groupe Européen de Curiethérapie – European Society for Radiotherapy and Oncology (GEC-ESTRO) guidelines [10-12]. Prescription points were set 15 mm along the tandem ring and 10 mm perpendicular to the tandem in the right and left directions. The revised American Brachytherapy Society (ABS) point A was obtained by drawing a line connecting the mid-dwell positions of the ring. Moving superiorly along the tandem 2 cm plus the thickness of the ring (including the cap), and then 2 cm perpendicularly to the tandem in the lateral direction, starting at the junction of this with the tandem [12]. These points were called ARN (right ABS new point) and ALN (left ABS new point). However, during the current study, these prescription points were named French prescription points (ARF: right French and ALF: left French). Furthermore, Manchester left and right points B (i.e., BL and BR, respectively) were also determined at 5 cm laterally from the midline, but at the level of ABS points A [5-14]. BT prescription dose was 25-35 Gy in 5 fractions. Prescription dose range was determined using linear quadratic equation (i.e., well-known total equieffective dose concept and EQD2 formula), with α/β of 10 for tumor and 3 for OARs. One applicator insertion was done in five subsequent treatment fractions for three days in a row. Patients were hospitalized, and at least 8 hours intervals were kept between each exposure. However, imaging was done before each fraction to confirm applicators’ and OARs positions. Inverse treatment planning with further manual optimization was performed for each patient, so that the 100% isodose curve covered the ring surface.
Organ at risk and target delineation
The bladder and rectum were contoured as OARs. OAR dose constraint was defined as 70-75 Gy and 90 Gy to D2cm3 (dose to maximum exposed 2 cm3 of organ volume) of the rectum/sigmoid and bladder, following EQD2 formula, respectively. Lymph node delineation was performed in compliance with latest comprehensive reference published by JCOG [15]. Moreover, to delineate obturator lymph node, a higher margin was applied by using an 18 mm brush, as explained in Taylor guidelines [16]. Another radiation oncologist double-checked all the targets, OARs, and lymph node delineation. All the determined points described above were positioned by an expert brachytherapy medical physicist to minimize inter-observer uncertainty (Figure 1). The approved plan was exported to GammaMed Plus HDR afterloader (Varian Medical Systems, Palo Alto, CA, USA).
Data extraction
Dose to points A and B as well as dose-volume histogram (DVH) parameters of delineated lymph nodes were extracted. Obtained DVH parameters of lymph nodes included D98 (dose to 98% of organ volume) as near-minimum dose, D2cm3 as near-maximum dose, and D50 (dose to 50% of organ volume) as organ median dose [17].
Statistical analysis
Descriptive analysis was performed and patients and treatment characteristics were recorded. T-test was used to assess for any significant relationship between the extracted data. Moreover, a correlation between these averaged differences and target volume were evaluated. A correlation between the extracted DVH parameters and the prescribed dose was studied through linear regression analysis and Pearson correlation tests by considering a statistical significance level of 0.05 (p-value < 0.05). Finally, correlations between DVH parameters of delineated pelvic lymph nodes and point B dose were assessed. IBM SPSS® statistics (version 23, IBM Corp.) software was used to perform statistical tests.
Results
Descriptive statistics
Thirty-six stage IB1-IIIB cervical cancer patients were included in the study. The median age was 60 years (range, 29-88 years), and CTVHR volume was 25.3 ±16.5 cm3. The descriptive data about their target and OARs doses are presented in Table 1. The mean radiation dose and mean percentage of prescription dose on the delineated lymph nodes are shown in Table 2. The mean dose and mean percentage of the prescription dose to the left and right B points were 4.6 ±0.18 Gy and 82.08 ±0.72%, respectively. The mean prescription dose to ARF and ALF points was 26.6 ±2.9 Gy for the patients.
Table 1
Descriptive statistics for patients DVH parameters
Table 2
DVH parameters of pelvic lymph nodes as well as the results of a correlation study between these parameters and prescription dose and mean dose of points B
T-test and correlation analysis
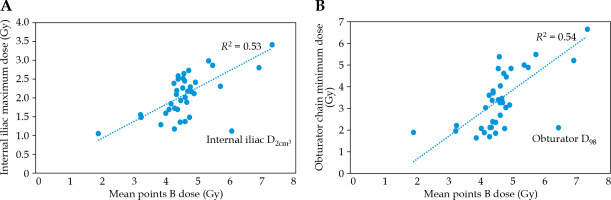
A Pearson correlation coefficient (R) R = 0.81 (p-value = 0.00) was obtained between the mean dose of the ARN and ALN points and the prescription dose. The p-value for paired-sample T-test between the dose to ARN and ALN points became 0.00. A negative correlation with R = 0.34 (p-value = 0.04) and RC = 0.2 was obtained between CTVHR volume and difference between French and ABS prescription points. Table 2 demonstrates the results of descriptive analysis of considered parameters. Figure 2 shows the maximum dose to the internal iliac and the minimum dose to the obturator chain with dose to points B. A Pearson correlation coefficient of R = 0.76 was found, with RC = 0.31 (p-value = 0.00) for correlation assessment between average dose to point B and prescription dose to each case. There was a low standard deviation between the dose to the left and right B points (SD = 0.18 Gy). Therefore, the correlation between the mean dose of B points and lymph node DVH parameters was calculated. These correlation results are also presented in Table 2.
Discussion
The results in Table 2 pointed out that there was some low to moderate correlation between the prescription dose and the dose to the pelvic lymph nodes. High standard deviation of the lymph nodes DVH parameters, especially their D2cm3, emphasizes the necessity to perform an individualized assessment of the dose to these organs. However, moderate to high correlations between DVH parameters of the pelvic lymph nodes and historical points B were shown. Initially, the absorbed dose to the left and right ICRU points B was expected to correspond to adjacent internal iliac and obturator lymph nodes absorbed dose [18]. In the present study, all pelvic lymph nodes were studied to determine if there was any correlation between dose to ICRU points B and other node groups. When Pearson correlation coefficients were between 0.5 to 0.7 and 0.7 to 0.9, correlation was interpreted as moderate and high, respectively. When the coefficient was positive, the correlation was termed direct, whereas negative correlation was termed indirect [19]. The correlation test results between the mean dose of the ARN and ALN points and the prescription dose indicate a positive and strong correlation. Paired sample T-test resulted in a significant (p-value = 0.00) relationship between the prescription dose and the ABS points dose. A negative correlation between CTVHR volume and difference between French and ABS prescription points means that by every 1 cm3 increase in target volume difference between these two definition strategies decreased by 0.2%, as expected due to increased ring radius and similarity in positions of two prescription points set. On the other hand, a high (R > 0.7) and significant correlation was obtained between points B and prescription. By every 1 Gy prescription dose, point B dose enhancement would increase by 0.31 Gy. However, despite the obturator lymph nodes, dose to point B underestimated the maximum dose to the pelvic node chain (Table 2). The maximum dose to the obturator nodes (range, 6.5-6.6 Gy) was about 40% higher than to the point B. The maximum dose was about 75% of the point B dose for the internal and external nodal chains. The most significant difference was for the common iliac group maximum dose, which was about 30% of the points B dose. The difference was higher between the minimum dose to these lymphatic chains and the B points. The higher minimum dose was observed to the obturator group, with about 50% of the point B dose. Point B dose (i.e., 4.6 ±0.18) was about three times the minimum dose to the internal and external iliac chain (i.e., D98 of these lymph nodes in Table 2), which would tend to support Gebara et al. study results [20]. Minor discrepancies can occur because they reported a higher minimum internal iliac dose and did not consider the near-minimum dose (D98) concept. Chua et al. also tried to quantify brachytherapy dose contribution to pelvic nodal groups following the Manchester system [6]. They prescribed the planning dose to Manchester point A and assessed the resulting mean pelvic lymph node dose. They concluded that the pelvic nodal groups received 15.9% (for the external iliac group) up to 30.1% (for the obturator group) of prescription dose. They did not consider pre-sacral and common iliac lymph nodes. D50 of obturator chains received the most absorbed dose in the current study, while they were only 15% of the prescribed dose to the ARF and ALF. This discrepancy was expected, because the distance between the prescription point and the pelvic lymph nodes in the current study was greater than the Manchester or ABS points. Dose to the internal iliac lymph nodes correlated with the prescription dose (R = 0.5-0.6). By every 1 Gy increase to prescription dose, about 0.35 Gy increase in near max dose of right and left internal iliac chains would be expected, referring to the obtained RCPD (Table 2). This correlation is moderate but statistically significant (p-value < 0.05). Pearson correlation coefficients of the near minimum and median dose indicated a moderate correlation (> 0.5) for the common iliac lymph nodes, but with a low RC. With every 1 Gy prescription dose growth, just a 0.15 Gy increase in these DVH parameters may occur. Other correlation tests between prescription dose and DVH parameters of the pelvic lymph nodes were weak and unreliable. A moderate and positive correlation (p-value = 0.00) was obtained between the dose to the pre-sacral lymph node and the point B dose. Previous related studies did not assess pre-sacral lymph nodes. According to Liu et al., metastatic pattern recurrence in this lymphatic chain is rare for locally advanced cervical cancer patients. However, these lymph nodes have been almost included in EBRT treatment fields [2]. Moderate to high and positive correlations were obtained between dose to internal iliac lymph node and mean dose of points B. For example, it can be interpreted that by every 1 Gy increase to prescription dose, their near maximum dose (D2cm3) may increase by about 1.1 Gy. However, there were some weak to moderate correlations between the external iliac DVH parameters and the mean dose to point B. Near minimum (D98) and median dose (D50) to the obturator lymph nodes presented moderate but statistically significant correlation with the points B dose. D2cm3 of these lymph nodes showed weak correlations (R = 0.34 and 0.36 for the left and right ones, respectively) to points B dose [18, 19]. In addition, there were weak correlations between common iliac lymph node DVH parameters and points B dose based on the present study results. However, Gebara et al. found good agreement between point B dose and maximum common iliac nodal dose in cervical cancer patients treated with Fletcher-Suit-Delclos applicator set [20]. Such contradicting results can be due to difference in applicator sets or point A definition protocol.
Lee et al. found that dose to point B and D2cm3 of the obturator lymph nodes and D0.1cm3 of the external iliac chain were not significantly different after evaluating the correlation between point B and pelvic lymph node dose in HDR brachytherapy of cervical cancer [5]. They only considered internal and external iliac chains and the obturator lymph node groups. D0.1cm3 was not assessed in the current study; nonetheless, there was a significant correlation between all retrieved lymph node DVH parameters, except the near minimum of external iliac chains. In the case of a follow-up or recurrence, the accumulative dosage to the lymph node delivered by EBRT plus brachytherapy may be of particular importance. Furthermore, when there is evidence of pathological lymph node involvement, paying attention to lymph node dosage is even more important.
Conclusions
Dose contribution to the pelvic lymph nodes during HDR brachytherapy is significant and must be managed carefully. The current study show that the dose to point B can be moderate to good surrogate for the maximum, minimum, and median dose to the internal iliac and pre-sacral lymph node, but cannot be for the maximum dose of obturator lymph node. Moreover, points B cannot be reliable substitutes for common and external iliac chains.