Purpose
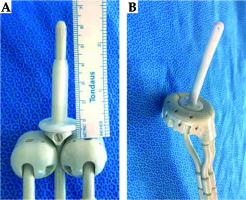
The Smit sleeve is a device developed by Elekta (Veenendaal, The Netherlands). It is a disposable, silicone-based indwelling intrauterine tube that can be used during brachytherapy treatment for cervical cancer to allow for easier insertion of the tandem [1]. The tube has a flange on the distal side, which acts as a stopper during tandem insertion [1,2,3,4]. The cannula of the Smit sleeve marginally increases the radius around the tandem, while the base of the Smit sleeve, which lies distal to the external cervical os, displaces the ovoids distally. In some tandem and ovoids systems, the flange on the tandem also lies between the cervix and the superior surface of the ovoids and must be accounted for when measuring the total displacement of the ovoids. In the tandem and ring systems, the base of the Smit sleeve fits in the opening of the ring and the use of this device has no impact on the geometry of the implant. The Smit sleeve on a tandem and ovoids model can be seen in Figure 1, where the combined thickness of the Smit sleeve and the flange is 5 mm, pushing the ovoids away from the target tissue. Distally displacing the ovoids by 5 mm may impact the dose delivered to the high-risk clinical target volume (HR-CTV), organs at risk (OARs), vaginal mucosa, and the integral dose (ID).
Fig. 1
Smit sleeve with a tandem and ovoids (A) compared to a tandem and ring (B). A) The distal displacement of the ovoids caused by the Smit sleeve, moving the ovoids away from the target. The use of a Smit sleeve with a tandem and ring applicators system does not have the same consequence on the applicators’ geometry
Previous studies have evaluated the dosimetric effects of applicator shifts and have found that shifts of a few millimeters can lead to large deviations in dose distribution, potentially leading to a shift in dose from the target volume to OARs [5,6,7,8]. One study, which retrospectively simulated tandem and ovoid shifts, found that a displacement by 3 mm cranially or caudally could cause greater than a 10% dosimetric change, showing that dose errors can occur when the reconstructed applicator position and actual applicator position differ [6]. These changes in dosimetric planning caused by tandem and ovoid displacements support the idea that the presence of the Smit sleeve may impact high-dose-rate (HDR) brachytherapy dose distribution.
To our knowledge no previous studies have assessed the impact of Smit sleeve usage on dosimetric planning. This technical note summarizes the dosimetric effects of the Smit sleeve on HDR brachytherapy tandem and voids plans by comparing plans in which a Smit sleeve was used to paired simulated plans in which the ovoids were shifted proximally to simulate removal of the device. HR-CTV coverage (D90) was kept the same between both plans set, by adjusting the dwell times of the simulated plan. The endpoints included the maximal doses delivered to 2 cc (D2cc) of the bladder, bowel, rectum, and sigmoid and to the International Commission on Radiation Units and Measurements (ICRU) rectal point, the latter of which was examined in response to sub-analysis of the EMBRACE study, which demonstrated that the ICRU rectal point is a predictive factor of grade 2 or higher vaginal toxicity, with grade ≥ 2 toxicity increasing from 20% to 34% when this point dose increased from 65 to 85 Gy EQD2 [9].
Material and methods
The brachytherapy plans of 11 patients with locally advanced cervical cancer (LACC) International Federation of Gynecology and Obstetrics Stages (FIGO) IB2-IVA who received HDR brachytherapy (Elekta’s microSelectron with iridium-192 [192Ir] source) with the use of a Smit sleeve and a tandem and ovoids system were identified through an institutional registry and selected for this dosimetric analysis. Brachytherapy applicators’ characteristics are listed in Table 1. Different tandem and ovoid sizes were used based on patient anatomy.
Table 1
Tumor and treatment characteristics
All treatment plans were volume-based, built on a planning pelvic CT scan (2.5 mm slice thickness) performed with the applicators in place. The treatment planning system utilized was the Oncentra Brachy Version 4.5.3 (Elekta, Veenendaal, The Netherlands). The applicators were reconstructed and the HR-CTV, bladder, bowel, rectum, and sigmoid were contoured by the treating radiation oncologist. The prescription dose to the HR-CTV was either four fractions at 700 cGy per fraction (n = 6) or three fractions at 800 cGy per fraction (n = 5). The target dose coverage of the HR-CTV was at least 90% for all plans and as high as 95% for some implants when the dose to the OARs allowed for increased HR-CTV coverage.
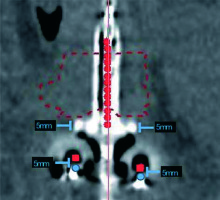
For the dosimetric analysis, the initial brachytherapy plans were modified to simulate removal of the Smit sleeve by shifting the ovoids proximally 5 mm, along the tandem. This process is displayed in Figure 2. The goal of this shift was to simulate a plan in which the Smit sleeve was absent, allowing for the ovoids to lie adjacent to the external cervical os. After shifting the ovoids as described above, the dose distribution was re-scaled by adjusting the dwell times uniformly so as to provide the same HR-CTV dose-volume coverage as the initial plan. As a result, the HR-CTV coverage (D90) was the same between the original and simulated plans. The relative dwell times were adjusted correspondingly although no re-optimization was performed.
Fig. 2
Coronal view of the planning CT of the Smit sleeve and tandem and ovoids delineating the simulated 5 mm proximal shift parallel to the flange of the Smit sleeve. Original source positions are displayed in blue and modified source positions are displayed in red
For each original HDR treatment plan and each modified plan, the integral dose, highest dose delivered to 2 cc (D2cc) of the bladder, bowel, sigmoid, rectum, and the ICRU rectum point dose were recorded. The ID was calculated by multiplying the source strength by the dwell time and represents the actual energy deposited in the tissue. In line with the ALARA principle, the ID to any uninvolved organ should be kept as low as reasonably achievable, as increased ID to normal structures near target volumes can result in toxicity or an increased risk for secondary malignancies. Thus, any change to integral dose is important to evaluate and was collected in this study. The dose delivered to the ICRU rectal point was used as a surrogate to evaluate vaginal toxicity [9]. The ICRU rectal point was placed during the retrospective modification of plans using the methods of the EMBRACE trial, and was reviewed by a board certified radiation oncologist [9].
The dose parameters captured were compared between the original and the modified plans using a paired two-sample t-test, with a p-value of < 0.05 considered statistically significant.
Results
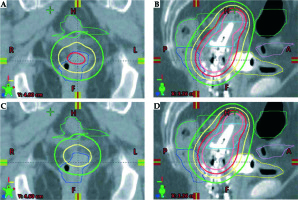
A total of 22 plans (11 original and 11 from reconstructed applicators’ geometry) were analyzed. Table 2 lists the mean difference in dose between the original and the modified plans for all variables and the results of the paired two-sample t-test. The integral dose required to achieve a dose coverage of the HR-CTV that matched that of the initial dose prescribed was reduced by a mean of 6.1% in the 5 mm shifted plan (p < 0.001). The mean rectum D2cc was 10.9% less in the modified plans when compared to the mean rectum D2cc in the original treatment plans (p = 0.004). The mean D2cc reductions for the remaining OARs and the ICRU rectum point ranged from 1.1% to 2.2%. Among these values only the mean D2cc reduction to the sigmoid of 1.8% was found to be significant (p = 0.04). The remainder were not found to be statistically significant. An illustration of the isodose modification observed on a given plan with and without the Smit sleeve in place is provided in Figure 3.
Table 2
Mean difference in integral dose and OAR dose between the original and modified plan. Modification included a 5 mm proximal shift of ovoid source positions parallel to the tandem
Fig. 3
A, B) Coronal and sagittal view of a plan with the sleeve present, no correction; C, D) coronal and sagittal view of a plan which mimics dosimetry without a Smit sleeve where ovoids were shifted 5 mm superiorly. The rectum, shown in blue, presents a notable reduction in volume receiving dose above the Rx dose (700 cGy red isodose line) in the plan mimicking a situation where the sleeve is not present
Discussion
In the present study, we quantified the impact of the distal shifts in the ovoids caused by the use of a Smit sleeve on the integral dose and the dose to OARs during endocavitary brachytherapy for LACC. The results of this dosimetric analysis confirmed that the Smit sleeve does have a dosimetric impact, which should be accounted for when a tandem and ovoids system is used for LACC endocavitary brachytherapy.
Numerous studies have evaluated the effects of source position shifts and obtained comparable results [6,7,10]. One study evaluating virtual tandem and ovoid shifts in the cranial and caudal setting found that a 3 mm shift of the applicator in the caudal direction resulted in a 10% change in the rectum D2cc, with similar lesser magnitude changes to the D2cc to the bladder, sigmoid, and ICRU rectal dose [6]. Another study which evaluated shifts in the x, y, and z axis and rotational shifts found that a 6 mm inferior shift increased the rectal dose by an average magnitude of 8.35% [10]. Furthermore, a study evaluating systematic applicator reconstruction uncertainties on dose volume histogram (DVH) parameters found that the rectal DVH shifted by approximately 3.5% per mm shift in the longitudinal direction and that of the HR-CTV, bladder and sigmoid colon shifted 1% to 2% per mm shift in the longitudinal direction [7]. Although our study focused on the ovoid, rather than the entire applicator, shifts, similar outcomes were observed.
The magnitude of change in the rectum D2cc was the most significant among the OARs. In cases where the Smit sleeve is utilized, caution should be taken to make sure the dose to the rectum is kept to a minimum [11]. Although the reduction in D2cc was marginal for the remaining OARs, these additional doses to OARs may be of relevance for plans which are close to recommended dose constraints. Practitioners should be cognizant of these effects and evaluate the patient’s anatomy prior to brachytherapy to determine whether distal displacement of the ovoids through the use of a Smit sleeve could impact the dose delivered to the OARs. In addition, they should consider using other techniques to reduce the dose to OARs such as speculum based vaginal packing [12], changes to bladder volume [13] or other techniques.
To conclude that the Smit sleeve is without benefit however would not be correct. Use of the Smit sleeve allows for easier insertion of the intrauterine tandem, decreasing the risk of uterine perforation, and provides an additional imaging marker to localize the cervix [11,14]. Given these benefits, providers may still elect to use the sleeve, either accepting the risk of a higher mean integral dose and OAR dose when used with a tandem and ovoids system. Moreover, if a Smit sleeve is necessary it might be better to consider using a tandem and ring applicator, since the Smit sleeve does alter the applicators’ geometry in this case, as can be seen in Figure 1.
The development of a modified Smit sleeve with a reduced base height or a different anchoring system which would reduce the displacement of the ovoids might be a solution.
Finally, preset applicator model templates are sometimes used during catheter reconstruction, especially with the growth of 3D image based treatment planning [15,16]. Use of templates in which the ovoids shift, caused by the presence of the Smit sleeve, this not being taken into consideration, could lead to inaccurate assessment of the doses delivered to the HR-CTV. As previously reported, any impactful variation in catheter positioning on simulation should result in manual reconstruction of the catheters in the treatment plan [7,17]. Given the variation caused by Smit sleeves, catheters with Smit sleeves should be manually reconstructed.
Although this dosimetric analysis conveys the importance of accounting for the Smit sleeve when planning HDR brachytherapy with a tandem and ovoids system, it has several limitations. This analysis did not account for the lateral displacement of tissue caused by the cannula of the Smit sleeve. Additionally, contouring of the HR-CTV and other structures can differ between providers, which can alter the dosimetry [18]. This possible variation was not accounted for in this study. Future studies should attempt to evaluate the dosimetric effects of the Smit sleeve through phantom studies and prospective evaluation.
Conclusions
Use of a Smit Sleeve with a tandem and ovoids system increases the integral dose and might affect the dose delivered to the OARs, particularly the rectum. Use of this device should be carefully considered when planning HDR brachytherapy with a tandem and ovoids system. A tandem and ring applicator might be a better choice for these clinical situations.



