Purpose
Endometrial carcinoma is one of the most common gynecologic tumors in the world [1]. Post-operative high-dose-rate brachytherapy with vaginal cylinder to irradiate the vaginal cuff (HDR-VCB) has established itself as adjuvant radiotherapy treatment of early endometrial cancer [2-4].
The goal of HDR-VCB is to reduce local recurrence rates in vaginal cuff, but it may increase normal tissue toxicity [5]. Therefore, it is important to measure the dose received by normal tissues and pelvic organs at risk (OARs) during treatment [6, 7].
Laparoscopic approach to routine procedure for endometrial cancer modifies the technique of suturing the vaginal stump, which can be performed transvaginally [8]. There is a possibility that small bowel loops may remain very close to, and even attached to the inner end of vaginal cuff. Higher doses may be received in HDR-VCB treatment due to the proximity of vaginal cylinder, which cause unwanted intestinal morbidity [9].
Currently, treatment planning with HDR-VCB is based on the use of CT after gynecologic application [10, 11]. In delineation of clinical target volume (CTV), the American Brachytherapy Society (ABS) and GEC-ESTRO (the Groupe Européen de Curiethérapie and the European Society for Radiotherapy and Oncology) recommendations are followed [12, 13]. Clinical target volume, which is considered the upper third portion of the vagina, is usually between 3 cm and 5 cm in length, with longer length in cases of adverse histology [6, 14, 15]. The bladder [16], rectum, sigmoid colon [13], and small bowel [13, 17] should be deemed as organs at risk within the pelvis. In current guidelines, there are no recommendations for the use of oral contrast in the small bowel during contouring planning [13]. After laparoscopic surgery, it would help to distinguish small bowel loops from the sigmoid and rectum. Regarding the rectum, enema before cylinder insertion does not influence recto-sigmoid dosimetric parameters [18].
Non-consensus for HDR-VCB
There is no consensus on several specific aspects when administering HDR-VCB treatment, such as bladder filling [19], placement of Foley catheter in the bladder [20, 21], amount of volume to fill the bladder [19, 21, 22], and procedure for a precise delimitation of the small bowel, the most radiosensitive organ relative to the bladder, rectum, and sigmoid [19, 23]. In patients undergoing laparoscopic surgery and HDR-VCB, administration of oral barium contrast offers the advantage of clearly defining small bowel loops proximal to the vaginal cuff to differentiate them from other bowel loops in order to evaluate dosimetric consequences in this OAR with different bladder filling. There are no published studies that assess dosimetric impact on OARs by modifying bladder filling in HDR-VCB treatment in laparoscopic surgery and oral barium contrast in the small bowel. Therefore, this was the aim of the present study.
It is worth highlighting that a novel aspect of this study is the use of oral barium contrast in the small bowel during CT treatment planning in HDR-VCB.
Material and methods
Patients
Between November 2019 and December 2020, 19 women were included in this prospective dosimetric study. Prior approval was obtained from the Hospital’s ethics committee. None of the patients had previously received HDR-VCB or external pelvic radiotherapy. Inclusion criteria were total laparoscopic hysterectomy (TLH) plus bilateral salpingo-oophorectomy and pelvic ± para-aortic lymphadenectomy, confirmed histology of endometrial carcinoma with no other cancer, age over 18 years, and good performance status. Each patient provided written consent to participate in the study.
Staging of the International Federation of Gynecology and Obstetrics (FIGO) 2009 and pathologic characteristics of the patients are shown in Table 1.
Treatment preparation and planning
All the patients received exclusively vaginal cuff HDR-VCB. Three hours before the procedure, oral barium contrast and rectal micro-enema were applied. At baseline, a detailed gynecologic examination was performed to evaluate the vaginal cuff and select the diameter of cylinder. A Foley catheter was then inserted with 7 ml of saline solution. Intracavitary HDR-VCB was performed with a magnetic resonance imaging (MRI), compatible plastic applicator with cylinder diameters between 2.5 and 3.5 cm. The applicator was fixed with CT/MRI applicator clamp (Elekta Company) with the cylinder positioned horizontally and legs extended, maintained throughout the treatment.
On the first day of treatment, two scans were performed successively, keeping the patient immobile in the same position: first scan with the bladder emptied and the second one with the bladder filled with 180 cc of saline solution and 5 cc of iodinated contrast, using a clamp holding the catheter. We used contrast in the bladder, following the same institutional protocol used in gynecological brachytherapy. The pelvis was scanned from the lumbar region to the ischial tuberosity. CT images were acquired with Siemens Somatom Confidence RT Pro scanner, with 120 KV and 1.5 mm thick slices.
Both sets of CT images were transferred to Oncentra® Brachy comprehensive treatment planning for brachytherapy system (Elekta Company, Veenendaal, The Netherlands). Vaginal length was measured on CT images.
The external contour of cylinder was delimited in the desired treatment length of 3-5 cm. The cylinder has not been excluded from CTV, but this did not influence the study, as the way the treatment was reproduced in empty and full bladder planning, making CTVs dose distributions identical.
A 5-mm expansion over the cylinder contour was performed automatically in all axes, except for the lower longitudinal, forming CTV. The dose of 7 Gy to CTV was prescribed for 3 cm and 3.5 cm cylinders, and 6.4 Gy for 2.5 cm cylinder, to administer the same equivalent dose (EQD2) to the cylinder surface. One fraction per week was administered. Total administered doses were 21.0 Gy and 19.2 Gy, respectively. Dose calculation in OARs was performed following ICRU 89 standards [24].
Plans were performed with Oncentra Brachy v. 4.5.3 planning system. First, the planning was carried out on empty bladder CT scan. For this purpose, dose points were generated at 0.5 cm from the cylinder surface, following CTV length. Then, the plan dose was optimized and prescribed, so that 100% doses were uniform at these points for source dwells positions set to 0.25 cm. Later, dwell positions and times obtained in empty bladder planning were reproduced on full bladder CT. Calculation algorithm was based on a TG43 formalism, which is the established standard for brachytherapy calculations, and does not consider density differences.
Finally, the treatment was delivered using an 192Ir gamma radiation source using Nucletron MicroSelectron Afterloader (Elekta Instrument AB; Stockholm, Sweden).
The dosimetric values were expressed as the minimum dose at 0.1 cc, 1.0 cc, and 2.0 cc volume (D0.1cc to D2cc) plus exposed OAR, and the dose received by 50% of OAR volume (D50%). For OAR volumes receiving 20% and 50% of the prescribed dose (V20 and V50 in cc), the dose was delivered at 5 mm from the cylinder surface. The prescribed dose was administered in the first fraction with the bladder previously emptied by Foley catheter, and in the two successive fractions with the bladder empty, without Foley catheter. The rest of sessions were conducted with an empty bladder, with the same dosimetry as set on the first day. Patients not included in this study were treated with an empty bladder using the same dosimetric protocol.
OARs contouring
The bladder, rectum, sigmoid, and small bowel were considered OARs, and were contoured as solid organs in 3 dimensions by one physician and checked by another. They were contoured on CT with empty bladder and with full bladder. The rectum was contoured from the anal sphincter to the sigmoid colon area, where the rectum loses its’ rounded shape in the axial plane and adopts a vertical orientation. It stops when the colon ascends laterally. The small bowel was contoured using barium contrast as an aid, up to 4 cm above the upper edge of the cylinder.
Statistics
The volumes of all specified OARs were determined, and dose-volume histograms generated in empty and full bladder states were compared. We planned the study with a continuous response variable from matched pairs. Prior data [21] indicated that the difference in the response of bladder D2cc dose pairs is normally distributed, with a standard deviation of 1.22 Gy. According to Guler et al., for a true difference in the mean response of 0.85 Gy in the bladder D2cc, we would need to study 19 patients for a significance level of 0.05 and statistical power of 0.8. Statistical evaluation was performed on dosimetric changes produced by anatomical changes, and all statistical analysis were assessed with SPSS software, version 21.0 (SPSS, Chicago, IL, USA). Graphics were made with Microsoft Excel 365®.
Results
The mean patient age of the 19 analyzed patients was 58 years (range, 38-81 years). Patients’ characteristics, including FIGO stage (2017), histology, tumor grade, surgery, cylinder size, and dose per fraction are shown in Table 1. The number of fractions treated was 3 in all patients. None of the patients experienced discomfort due to Foley catheter or cylinder insertion. The mean full bladder volume was approximately four times larger compared to empty bladder (248.54 cc vs. 63.77 cc, p < 0.001). With an empty bladder, the small bowel generally moved anteriorly, laterally, and superiorly, as presented in Figure 1.
Fig. 1
Organs at risk (OARs) in axial with full bladder (A) and sagittal with full bladder (B) (small bowel with barium contrast displaced anteriorly, superiorly, and laterally); in axial with empty bladder (C) and sagittal direction with empty bladder (D) (small bowel loops with barium contrast located very close to the cylinder)
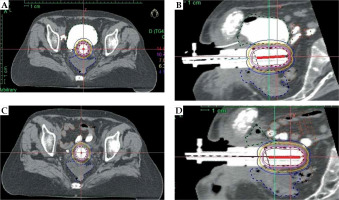
Bladder distension did not have a significant dosimetric impact on the bladder with D2cc from 5.06 to 5.56 Gy (p = 0.07), nor on the rectum or sigmoid, but it did have a significant impact on the small bowel, with D2cc from 2.70 Gy to 1.68 Gy (p < 0.001) and D50% from 1.09 to 0.70 Gy with a full bladder (Table 2). D50% of the bladder was affected by bladder distension from 1.28 to 2.11 Gy, p < 0.001. In Figure 2, D2cc and D50% values for each individual patient are displayed the for bladder in Figure 2A and B, and for the small bowel in Figure 2C and D.
Fig. 2
Values D2cc (Gy) and D50% for individual case. Dashed line: empty bladder, red line: full bladder. A) D2cc bladder; B) D50cc bladder; C) D2cc small bowel; D) D50cc small bowel
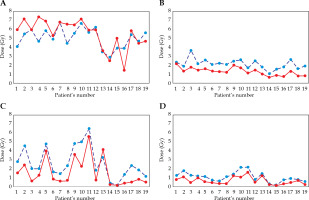
Table 2
Mean dosimetric values of bladder and small bowel in full and empty bladder filling
Discussion
In our study, the dosimetric effect of bladder filling in HDR-BT treatment with distended bladder significantly reduced the highest dose in the small bowel proximal to the vaginal cuff, visualized with oral barium contrast, with no significant change in the bladder, rectum, or sigmoid dose. To our knowledge, this is the first prospective study using barium contrast for small bowel delineation in post-operative HDR-BT treatment of vaginal cuff in patients operated by laparoscopy, facilitating differentiation with other bowel loops.
There are few studies [19-23] comparing dosimetric influence of bladder filling during HDR-VCB planning in OARs, and there are no studies quantifying a difference between using or not using contrast in the small bowel. All published studies are presented in Table 3.
Table 3
Authors and studies on dosimetric impact of bladder filling in OARs
| Autor, year [Ref.] | No. of patients,type of tumor,type surgery | Foley catheter | CT (empty and full bladder);use of oral contrast | No. of doses and fx. | Length of vagina treated | Organ at risk analyzed | Dosimetric and volumetric parameters | Results,full vs. empty bladder | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Hoskin et al., 2000 [22] | 30 patients, gynecologic carcinoma,not specified | Yes | 1. Rx pa. and lateral with catheter with balloon, and hypaque and barium in rectum2. Serial CT scan (5 mm) with empty and full bladder with 35 cc, 70 cc, and 100 cc; no contrast | 5.5 Gy at 0.5 cm × 4 fx. | 1/3 upper vagina | Bladder, small bowel | Bladder volume, bladder area, small bowel area, bladder mean max. dose, bladder height | Bladder dose not affected by volume;small bowel volume within high-dose treatment region decreased 57.5% with 100 ml bladder volume | VCB should be undertaken with a bladder volume of at least 100 ml, which will considerably reduce the amount of small bowel in irradiation volume, with no increase of the bladder dose |
| Stewart et al., 2008 [19] | 20 patients, gynecologic carcinoma,not specified | No | 1. fx.: bladder comfort filling2. fx.: full bladder with 950 cc3. fx.: empty bladder,no contrast | EBRT + BT (6 Gy × 2 fx.)orBT only (6 Gy × 5 fx.) | Upper half of the vagina (mean, 7 cm) | Bladder, rectum and urethra, maximum bladder point (MBP), maximum rectal point (MRP) | Bladder: V70cc, V50cc, surface area: 50 cc, D2cc,MBP, cc, and Gy;Rectum: D2cc,MRP, cc, and Gy | Bladder V70, V50, SA50, and D2cc were significantly lower for the empty bladder than for the full bladderBladder filling did not alter the volume or surface area of rectum irradiated | Patients receiving HDR-VCB should be treated with an empty bladder if feasible. MBP correlates well with volumetric assessments of the bladder dose and provides a non-invasive method for reporting MBP using 3D imaging |
| Hung et al., 2011 [23] | 12 patients, endometrium, cervix and ovarian, primary or recurrence,not specified | Yes: 180 cc | Yes: empty bladder and full 180 cc, no contrast | 2-5 fx., 5 Gy at 0.5 cm | Not specified | Bladder, rectum, sigmoid, small bowel | D2cc, D50%, ICRU points, cylinder-to-bowel distance | Full bladder: D2cc in the mean small bowel significantly decreased, and the bladder did notFull bladder: D50% decreased for the bladder and small bowelNot for rectum and sigmoid | Distended bladder preferentially reduced high-dose to the small bowel around the vaginal cuff without a significant change in dose to the bladder, rectum, or sigmoid |
| Kobzda et al., 2014 [20] | 45 patients (36 patients: only VCB), all stages endometrium, not specified | No previous BT, 400 ml intake | Yes: CT-full bladder, and after CT-empty bladder after (post-urinating),not contrast | 4 Gy × 6 fx. or 6 Gy × 3 fx. | 1/3 upper vagina | Bladder, rectum, sigmoid, small bowel | D0.1cc, D2cc to bladder rectum and bowel, V50%, V80%, EQD2 of OAR | D0.1cc and D2cc of the bladder were lower in the empty state, V50% and V80% were to be higher in the empty state | The dose to the empty bladder was lower than when the bladder is full. Protection of more radiosensitive bowels suggested treating the patients in the full state of the bladder |
| Guler et al., 2014 [21] | 15 patients, early stage endometrial cancer, total abdominal hysterectomy | Yes: 180 cc | Yes: CT-empty bladder and CT-full bladder (180 cc)Not contrast | 5 Gy × 5 fx. | 3-5 cm of vagina | Bladder, rectum, sigmoid, small bowel | D0.1cc, D0.2cc, D0.5cc, D1cc, D2cc; D50% | Bladder distention appreciably impacted dosimetry of the bladder, sigmoid colon, and small bowel, but dosimetry of the rectum was unaffected | The combination of a full bladder and an empty rectum may cause significant unwanted increases in VCB dosing of the bladder, without significantly impacting sigmoid colon and small bowel exposures; Better empty bladder |
| Salas et al., 2021[present study] | 19 patients, early-stage endometrial cancer, total laparoscopic hysterectomy | Yes: 180 cc | Yes: CT-empty bladder and CT-full bladder (180 cc); contrast in the bladder, and oral barium contrast in small bowel | 7 Gy × 3 fx. | 3 cm of upper vagina (endometrioid), 5 cm of upper vagina (serous) | Bladder, rectum, sigmoid, small bowel | D0.1cc, D1cc, D2cc; D50%, V20%, V50% | Bladder D2cc was not significantly higher with the full bladderV50% was lower;small bowel D2cc and D50% were higher with the empty bladder; V50% dose was not affected by the volume | Full bladder reduced high-dose to the small bowel during HDR-VCB with no significantly increased doses in the bladder, and not impacted the rectum or sigmoid;Treatment with a full bladder was advisable |
[i] OARs – organs at risk; Rx – X-ray; CT – computed tomography; VCB – vaginal cuff brachytherapy; HDR-VCB – high-dose-rate vaginal cuff brachytherapy; cc – cubic centimeters; fx. – fraction; EBRT – external beam radiation therapy; BT – brachytherapy; Gy – Grey; D0.1cc – dose to 0.1 cc of organ; D1cc – dose to 1 cc; D2cc – dose to 2 cc; D50% – dose to 50% of volume; V20% – percentage of organ volume receiving ≥ 20% of the prescribed dose; V50% – percentage of organ volume receiving ≥ 50% of the prescribed dose; V70% – percentage of organ volume receiving ≥ 70% of the prescribed dose; V80% – percentage of organ volume receiving ≥ 80% of the prescribed dose; ICRU – International Commission on Radiation Units; MBP – maximum bladder point; MRP – maximum rectal point; SA50 – surface area of bladder receiving ≥ 50% of dose; EQD2 – equivalent dose in 2 Gy per fraction
Hoskin and Vidler are the first authors to perform a comparative analysis of empty and full bladder. They based their measurements on a 2D bladder height, without evaluating volumetric parameters of the bladder. They found that instillation of 100 ml of water into the bladder decreased the amount of small bowel in high-dose region by 57%, without significantly increasing the mean maximum bladder dose. Full bladder treatment with at least 100 cc is recommended [22].
Steward et al. investigated dose parameters in CT planning with empty and full bladder on images acquired 1 hour after consumption of 32 oz of water. They reported that bladder distension increased bladder D2cc, with no appreciable change in rectal D2cc. Sigmoid colon and small bowel dosimetrics were not evaluated. In contrast, our result showed that bladder D2cc was +10% change, but not significantly higher with a full bladder. As the small bowel was not considered, we could not compare data in this OAR [19].
Hung et al. evaluated OARs doses to the rectum, bladder, sigmoid, and small bowel without a contrast. D2cc (1.179 cGy vs. 1.246 cGy, p = 0.11) and D50% (441 cGy vs. 279 cGy, p = 0.001) of small bowel with bladder filled with 180 cc via Foley catheter, resulted in a significant dose reduction with no significant changes in the bladder, rectum, or sigmoid. Our dosimetric calculation is in agreement with these findings, although the dose recorded at 2 cc bladder with full bladder was slightly higher, it was not significant. In addition, the dose to the small bowel was significantly lower with a full bladder. Their study was performed without oral contrast in small bowel. The authors advised treatment with distended bladder [23].
In a study by Kobzda et al., the bladder was filled with a 400 ml, ingested 40 min before CT. They described that bladder D2cc significantly decreased with empty bladder (4.9 Gy vs. 4.6 Gy, p < 0.05), while volumetric doses (V50 and V80) were significantly higher in the empty organ. Our results show a similar trend, but without statistical significance. This difference could be explained by a different volume of bladder filling used (400 cc vs. 180 cc). In the small bowel, it was associated with a significant reduction of the intestinal dose of D2cc (4.1 Gy vs. 4.6 Gy, p < 0.05). Their findings are consistent with the present results. Moreover, the authors recommended treatment with a full bladder [20].
In a study, Guler et al. assessed patients who underwent initial CT with an empty bladder and then, sterile saline (180 ml) was infused through a Foley catheter. Two-thirds of the vaginal cuff was treated. OARs, such as the bladder, rectum, sigmoid, and small bowel without contrast were analyzed. Full bladder produced a significant 18.7% increase in D2cc in the bladder (5.40 Gy vs. 4.55 Gy, p < 0.05), but not in the rectum. The reduction in sigmoid and small bowel was not significant. The results of our study are not consistent with these findings. One cause could be that their treatment activated two thirds of the vaginal cylinder, while in our protocol, only one third was activated. The other also did not use contrast in the small bowel [21].
Our dosimetric study has some limitations, including small number of cases, which makes it difficult to generalize the results. This was a dosimetric study that did not intend to draw clinical conclusions. The obtained data should be correlated with short- and long-term morbidity analyses, quality of life questionnaires, and in vivo dosimetry studies. Such a correlation could help to assess the doses to OARs, suggesting further research development.
Conclusions
In patients undergoing laparoscopic surgery for endometrial cancer treated with HDR-VCB, full bladder reduces a higher dose to the small bowel previously visualized with oral barium contrast, without a clinically relevant dose increase in the bladder and no dose increase in the sigmoid or rectum. Treatment with full bladder appears more beneficial dosimetrically due to possible impact on this risk organ.



