Purpose
Cervical cancer is the fourth most prevalent malignancy worldwide, with approximately 570,000 new cases and 311,000 deaths reported globally in 2018 [1, 2]. Concurrent cisplatin-based chemoradiotherapy followed by high-dose-rate (HDR) brachytherapy is considered the standard therapeutic approach for patients with stage IB3-IVA cervical cancer [3]. For locally advanced cervical cancer, brachytherapy involves intra-cavitary brachytherapy (ICBT), interstitial brachytherapy (ISBT), or a combination of both (IC + IS BT). An overall treatment time (OTT) exceeding 8 weeks was linked to poorer survival rates in patients with cervical cancer [4, 5]. This highlights the need for timely and effective treatment strategies to improve patient outcomes.
The respiratory syndrome, coronavirus disease 2019 (COVID-19), was initially identified in Wuhan, Hubei Province, China, in late 2019. In response, the Chinese government implemented a nationwide lockdown in January 2020, which was subsequently lifted in phases. Despite the impact of the COVID-19 pandemic on all hospital services, it is crucial to maintain brachytherapy as an indispensable component of cervical cancer treatment for improving clinical outcomes and extending patients’ life expectancy. Consensus guidelines recommended a modified approach with fewer applicator insertions and fractionated treatments to complete brachytherapy within a designated timeframe, accounting for potential delays [6-8].
Our institution adopted an abbreviated brachytherapy schema, in which the entire course of brachytherapy (7 Gy × 4 fractions) was administered over 10 days with 2-day intervals to address clinical demands, instead of the standard schedule of 4-5 fractions delivered over 4-5 weeks. This schema was implemented from February 2020 until the release of new guidelines in September 2020. In this paper, we reported on the clinical treatment data and follow-up outcomes of patients treated with this abbreviated brachytherapy protocol during the first year of the COVID-19 pandemic.
Material and methods
Patient population
Institutional Review Board (IRB) approval was obtained for the retrospective analysis and outcome reporting of abbreviated brachytherapy treatment schedules for cervical cancer. Medical records of patients with cervical cancer, who received CT-guided, three-dimensional brachytherapy between February and September 2020 were reviewed. Pre-treatment assessments included medical history, gynecologic examination, complete blood count, liver and renal function tests, chest radiography or contrast-enhanced CT, and pelvic MRI. Cystoscopy and sigmoidoscopy were performed selectively in patients with radiological signs of the bladder or rectal involvement. This detailed pre-treatment evaluation ensured that patient selection and treatment planning were tailored to individual’s clinical presentation and risk factors.
External beam radiotherapy and chemotherapy
Patients underwent external beam radiotherapy (EBRT) at a dose of 45 Gy in 25 fractions using intensity-modulated radiotherapy (IMRT). Those with positive nodal disease received nodal boosts via simultaneous IMRT-integrated boost (SIB), achieving a cumulative dose of 60 Gy in 28 fractions. A 6 MV photon beam was used for EBRT. Additionally, patients received cisplatin-based chemotherapy at a weekly dose of 40 mg/m2 during radiotherapy course. Chemoradiotherapy was paused for 1 week until adverse events resolved to the following criteria: Karnofsky performance status (KPS) ≥ 70, temperature < 38°C, absolute neutrophil count (ANC) ≥ 1,000/mm3, and platelet count ≥ 75,000/mm3 or higher. For grade 4 hematologic toxicity, complete blood counts were monitored more frequently, and treatment was suspended if necessary. This cautious approach ensured patient safety, while maximizing therapeutic efficacy.
Brachytherapy
All patients were deemed eligible for a 7 Gy × 4 fraction brachytherapy (BT) regimen, typically administered within a 10-day window following the completion of EBRT. BT sessions were meticulously scheduled on specific days, i.e., 1, 4, 7, and 10. Clinical team adopted this abbreviated schedule in response to patients’ difficulties adhering to weekly fractionated brachytherapy treatments during the COVID-19 lockdown period. Upon hospital admission, patients were required to undergo a comprehensive chest CT scan, reverse transcriptase polymerase chain reaction (RT-PCR) test for COVID-19, and epidemiological investigation. Patients were considered eligible for brachytherapy only if the above assessments revealed no abnormalities. All patients underwent bowel preparation with an enema the evening before and in the morning of brachytherapy procedure to ensure optimal treatment conditions.
CT-guided HDR brachytherapy was performed for all patients under general anesthesia. Patients were generally treated with intra-cavitary tandem and ovoid set using interstitial metal needles placed into the gross parametrial or cervical disease. Patients with central or medial parametrial involvement underwent ICBT, whereas cases with persistent lateral parametrial involvement received a combination of intra-cavitary and interstitial brachytherapy (IC + IS BT). High-risk clinical target volume (HR-CTV) was delineated based on MRI findings and clinical examinations before and after EBRT, following the GEC-ESTRO guidelines [9]. Organs at risk (OARs) included the bladder, rectum, sigmoid colon, and small intestine. Brachytherapy plans were developed using Oncentra planning system (version 4.5, Elekta Medical Systems, Sweden) and hybrid inverse planning optimization (HIPO), delivered with a Flexitron HDR afterloader (iridium-192 radioactive source, Elekta Medical Systems, Sweden). This meticulous approach to treatment planning and delivery ensured precise application of radiation doses to target volumes, while minimizing exposure to critical structures.
Following the completion of day 1 brachytherapy, the IC applicator and IS needles were promptly removed, and patients were transferred to a dedicated unit within a non-COVID inpatient ward. This unit was equipped with specialized nursing care and medical staff for optimal patient support. The inpatient order set included necessary medical support, but family visits were restricted. Patients were transported to radiotherapy department 1 hour before each scheduled brachytherapy session, where they underwent the same preparatory procedures as in their initial session, including applicator implantation, CT scanning, contouring, and treatment planning. Cumulative equivalent dose in 2 Gy per fraction (EQD2) dose goal for HR-CTV irradiation was ≥ 85 Gy EQD2 for α/β = 10 Gy. Concurrently, it was imperative to ensure that minimum doses delivered to 2 cc volumes of the bladder (B2cc), rectum (R2cc), and sigmoid colon (S2cc) did not exceed tolerance limits in EQD2 for α/β = 3 Gy at B2cc ≤ 90, R2cc ≤ 75, and S2cc ≤ 75 Gy, respectively. This approach aimed to balance the therapeutic efficacy with safety profile, ensuring that the provided treatment was effective and tolerable for patients.
Follow-up and evaluation of toxicity
After treatment completion, all patients underwent follow-up assessments for the first month, followed by evaluations every 3 months for the first 2 years, and then semi-annual checkups during the third and fifth years. At each follow-up visit, a comprehensive medical history, clinical examination, and various blood tests were conducted. MRI scans of the abdomen and pelvis were performed during the 3-month follow-up visit, and subsequently at the 6-month visit. Tumor responses were classified according to response evaluation criteria in solid tumors (RECIST, v. 1.1) criteria [10].
Patients were continuously monitored during the treatment period and for up to 90 days after treatment for acute gastrointestinal, rectal, genitourinary, and hematologic toxicities, according to the Radiation Therapy Oncology Group (RTOG) standards [11]. Medical records were reviewed to extract the disease control data. Time-to-event analysis reported oncological endpoints, such as progression-free survival (PFS) and overall survival (OS). All outcomes were calculated from the date of diagnosis to the date of relapse or last follow-up, as applicable. Deaths from any cause were considered as events in disease-free survival analysis.
Evaluation of outcomes and statistics
Progression-free survival was calculated from the start of concurrent chemoradiation therapy to the first documented disease progression event. OS was determined from the start of concurrent chemoradiation therapy until death from any cause. Patients who did not experience any event or were lost to follow-up were censored at the last documented follow-up visit. Estimates of PFS and OS were derived using Kaplan-Meier method. All data analyses were conducted using SPSS software (version 17.0, SPSS, Chicago, IL, USA).
Results
Sixty-nine patients with cervical cancer underwent radical chemoradiotherapy between February and September 2020. The characteristics of the patients are summarized in Table 1. The median age was 54.9 years (range, 36-78 years). According to the FIGO 2018 staging system, 14 (20.3%) patients had stage IB2-IIA disease, 20 (29.0%) had stage IIB, 3 (4.3%) had stage IIIA, 29 (42.1%) had stage IIIC1-C2, and 3 (4.3%) had stage IVA.
Table 1
Patient and treatment characteristics
All patients underwent pelvic EBRT, receiving a total dose of 45-50.4 Gy in fractions of 1.8-2 Gy, and 34 patients with positive nodes underwent a nodal boost. Eighteen patients underwent ICBT, whereas 51 received IC + IS BT. The treatment plan aimed to deliver a cumulative dose of at least 85 Gy (EQD2, α/β = 10 Gy) to HR-CTV but minimizing doses to OARs, i.e., the bladder, rectum, and sigmoid colon. The mean D90 for HR-CTV was 91.9 Gy (range, 84.8-102.9 Gy), with D2cc doses for the bladder, rectum, and sigmoid colon being 85.4 Gy (range, 75.8-90.9 Gy), 69.6 Gy (range, 56.8-76.3 Gy), and 66.5 Gy (range, 54.4-75.8 Gy), respectively. Concurrent chemotherapy was administered to 63 patients with 40 mg/m2 weekly cisplatin.
The median OTT for patients who completed therapy was 56.2 days (range, 45-73 days). Prolonged OTT, defined as > 60 days, was observed in 15 patients (21.7%), potentially due to delayed referral for brachytherapy in five cases (7.2%), and radiation-related adverse effects in the remaining 10 cases (14.4%). Acute grade 3 hematological toxicity occurred in 62.3% of patients, and grade 4 toxicity in 11.6%. Gastrointestinal grade ≥ 2 and ≥ 3 toxicities were 5.8% and 2.9%, respectively. Rectal and genitourinary grade ≥ 2 toxicities were 2.8% and 4.3%, respectively. Table 2 summarizes the acute hematologic and non-hematologic toxicities.
Table 2
Acute hematological and non-hematologic toxicities in patients
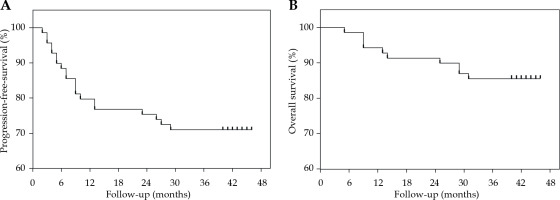
As of December 2023, the median follow-up duration was 40 months (range, 5-46 months). The patient outcomes are detailed in Figure 1. The 1-year local control, PFS, and OS rates were 97.1%, 81.2%, and 94.2%, respectively. The 3-year local control, PFS, and OS rates were 88.4%, 71.0%, and 85.5%. There were no treatment-related deaths. The cumulative incidence of grade 3 rectal complications was 2.9%, with no grade 4 rectal complications. Only one case of grade 4 bladder complications was reported. Late gastrointestinal toxicities of grade ≥ 2 and grade ≥ 3 occurred in 14 (20.3%) and 3 (4.3%) patients, respectively. Late genitourinary toxicities of grade ≥ 2 and grade ≥ 3 occurred in 8 (11.6%) and 3 (4.3%) patients, respectively.
Discussion
High-dose-rate brachytherapy plays a pivotal role in the treatment of cervical cancer, particularly in patients with locally advanced disease, in whom achieving local control and enhancing survival rates are crucial. The European Society for Medical Oncology (ESMO) guidelines recommend a prescription dose of 80-90 Gy for cervical cancer radiotherapy [12]. According to the 2012 evidence-based guidelines from the American Brachytherapy Society, the D90 for HR-CTV should be no less than 80 Gy. For patients with tumors ≤ 4 cm in diameter who exhibit an inadequate response to downsizing or for those with tumors > 4 cm, the dose should be escalated to 85-90 Gy. The GEC-ESTRO guidelines and the EMBRACE II study protocol adopt similar principles, proposing a model that correlates tumor volume, dose, and therapeutic effect for locally advanced cervical cancer cases [13, 14]. The EMBRACE II study suggests that for patients with a tumor volume exceeding 30 cm3, an additional increasing dose to HR-CTV is recommend [15].
The COVID-19 pandemic has significantly disrupted global healthcare services, necessitating adjustments to many treatment protocols to accommodate a new medical context [16, 17]. Conventional brachytherapy protocols typically require multiple implantations and fractionated treatments. However, under the unique conditions of the COVID-19 pandemic, these approaches face challenges, including unequal distribution of medical resources, restrictions in patients’ mobility, and protracted treatment durations. Comprehensive protective strategies are necessary to ensure patient safety. Patients should undergo rigorous screening and adhere to universal precautions, whereas suspicious cases must be managed according to international guidelines. Pre-operative preparations have to include clear risk disclaimers and efforts to minimize hospital stay. Healthcare workers must follow rigorous disinfection protocols and wear appropriate personal protective equipment, and the treatment area must be zoned to minimize the risk of cross-infection. During treatment, patients should be regularly monitored with weekly hematological evaluations. Post-treatment, patients need to focus on maintaining a balanced diet, adequate rest, and moderate exercise as well as on prioritizing self-protection and following quarantine protocols [6-8]. Consensus guidelines recommend reducing applicator insertions and using multiple fraction treatments to minimize repeated anesthesia procedures. Various institutions adopted different brachytherapy plans, such as an abbreviated brachytherapy schema, where patients receive a single insertion and multiple fractions (range, 3-5) brachytherapy over 24-48 hours, or a single-application schema, where patients are admitted to the hospital for two consecutive nights and receive either three daily (8 Gy) or five bis in die (BID) fractions (range, 4.5-5.5 Gy) [18, 19].
To alleviate clinical demands, minimize hospital visits, and reduce OTT during the pandemic, thus mitigating the risk of COVID-19 infection and ensuring therapeutic efficacy, our institution modified brachytherapy protocol to an abbreviated schema (beginning in February 2020) based on available infrastructure and resources. Applicator placement and CT guidance remained the same as pre-COVID; however, patients were hospitalized for 10 days and received a total of 4 HDR-BT fractions with a 2-day interval.
The current study aimed to evaluate the feasibility and safety of the abbreviated schema introduced during the COVID-19 pandemic through a comprehensive analysis of preliminary outcomes. This schema required completing the entire brachytherapy treatment within 10 days, significantly shortening the treatment duration. Our findings indicate that this condensed schema, while preserving therapeutic efficacy, decreased patients’ OTT, aligning with literature suggesting that an OTT exceeding 8 weeks could negatively impact survival rates in patients with cervical cancer. Furthermore, we demonstrated that even with an abbreviated schema, patients can achieve high local control and survival rates: 1-year PFS rate was 81.2% and 1-year OS rate was 94.2%, with 3-year PFS of 71.0% and 3-year OS of 85.5%. Our institution conducted a study between 2011 and 2017 on the efficacy of the single-channel and Fletcher applicators in brachytherapy for cervical cancer, following a standard delivery schedule of 5 to 6 fractions of 7 Gy each, administered once weekly [20]. This study reported 2-year OS and PFS rates of 80.3% and 77.5%, respectively, for the Fletcher group, and 86.3% and 82.5%, respectively, for the single-channel group. The severity of acute treatment-related toxicities was comparable between the two groups. The cumulative rate of late rectal complications (grades, 3-4) was 2.8% for the Fletcher group, and 2.5% for the single-channel group. The cumulative rate of grade 3 bladder complications was 2.8% for the Fletcher group, and 1.3% for the single-channel group. When compared with literature, our study on the abbreviated brachytherapy schema suggests that it does not compromise patients’ outcomes.
Safety is a paramount criterion in assessing the efficacy and risks for any treatment protocol. The abbreviated schema must be implemented without compromising treatment quality. CT-guided HDR brachytherapy targets radiation dose to tumor tissue, ensuring maximum safety of the surrounding normal tissues. This precise radiotherapy technique is an essential protection, facilitating the adoption of an abbreviated treatment schema. The incidences of acute grade 3 hematological toxicity (62.3%) and grade 4 toxicity (11.6%) were within the expected ranges for patients with cervical cancer undergoing intensive chemoradiation therapy. Rates of grade 3 or higher gastrointestinal and genitourinary toxicities were also acceptable, further supporting the safety of the proposed approach. These findings are consistent with an agreement that HDR brachytherapy when precisely delivered, can be safely administered with minimal additional toxicity [21, 22]. Despite the low incidence of toxicity, continuous monitoring remains imperative. Damast et al. highlighted a higher rate of mucosal toxicity in patients receiving a single application of IC + ISBT, underscoring the need for rigorous toxicity assessment and management when using abbreviated protocols [18].
Successful implementation of an abbreviated brachy-therapy schema depends on collaborative efforts of health-care team and patient adherence to treatment protocol. The COVID-19 pandemic has placed significant emphasis on medical resources, necessitating the optimization of treatment processes to minimize non-essential therapeutic interventions. Patient comprehension and active participation are essential for an effective treatment. This underscores the importance of clear communication and education to ensure that patients are well-informed about their treatment options and expectations from an abbreviated protocol.
Although the presented abbreviated brachytherapy schema shows promise, there are several limitations to acknowledge. First, the unique conditions of the pandemic may have resulted in the recruitment of insufficient sample size, potentially limiting the detailed assessment of long-term outcomes and late toxicities. Second, this abbreviated schema might not be universally applicable, as it may not accommodate patients’ individualized treatment requirements, particularly those with more intricate treatment needs. Furthermore, implementing an abbreviated treatment schema may demand additional resources and technical expertise to ensure the maintenance of treatment quality and safety standards. This highlights the necessity for careful consideration and customization of treatment protocols to address the diverse requirements of a patient population, while maintaining the highest standards of care.
Conclusions
During the COVID-19 pandemic, the presented abbreviated brachytherapy schema offered a viable alternative for patients with cervical cancer. Despite certain challenges, preliminary findings indicate that this schema can preserve therapeutic efficacy and safety without prolonging treatment duration. However, further research is essential to evaluate its long-term impacts and broader applicability. We are confident that with continued evidence and refinement of therapeutic strategies, the abbreviated brachytherapy schema will offer new hope to patients with cervical cancer. This highlights the potential for innovative approaches to improve patient care during crises, while emphasizing the importance of ongoing care and adaptability in medical practice.