Purpose
According to the Groupe Européen de Curiethérapie (GEC), the European Society for Radiotherapy & Oncology (ESTRO), and the American Brachytherapy Society (ABS), the applicator inclusion in considered clinical volumes should be mentioned when reporting dose-volume histogram (DVH) parameters [1, 2]. The volume of applicator does not significantly influence the parameters, if it is small compared to target volumes. Currently, there is no consensus on whether and how to deal with applicator’s volume in dosimetric evaluation. Chakrabarti et al. in their study included tandem in CTV contour on plan comparisons of different applicators [3]. Vivekanandan et al. excluded the needle contours in interstitial prostate high-dose-rate (HDR) brachytherapy. They reported average changes of less than 3% in PTV V100% (volume that received 100% of the prescription dose), V150%, and V200% after applicator exclusion [4]. Desbiens et al. excluded the contour of Syed-Neblett template with 6F needles and 2 cm cylinder. They reported a maximal change in CTV D90% (dose received by 90% of the volume) of 7.4% after applicator exclusion [5].
The Venezia applicator (Elekta, Sweden) can improve lower vaginal and lateral parametrium dose coverages for patients with locally advanced cervical cancer [6-12]. Walter et al. and Xu et al. reported high-risk clinical target volume (HR-CTV) D90% of 90 Gyα/β = 10 to 95 Gyα/β = 10, HR-CTV D98% more than 75 Gyα/β = 10, and intermediate-risk clinical target volume (IR-CTV) D98% more than 60 Gyα/β = 10, and all D2cc of critical structures met the ABS and EMBRACE II constraints [1, 13, 14]. The supplementary components make the overall applicator volume large compared to targets. Typically contoured volumes of HR-CTV and IR-CTV were 30 cm3 to 50 cm3, and 90 cm3 to 150 cm3, respectively [15-17]. However, the physical volume of vaginal caps varies from 55 cm3 to 90 cm3. When such applicator components are used, DVH parameters, for which small volumes are considered (e.g., D50%), may include an excess of applicator volume to be meaningful [2]. In addition, changes in high-dose DVH parameters, such as V150% and V200%, may increase with large applicator volumes. As a result, it is important to evaluate the dosimetric effect of applicator removal for Venezia applicator.
Material and methods
Patients and treatment
This study included 40 cervical cancer patients (101 plans) treated with whole pelvis external beam radiation therapy (EBRT) of 45 Gy in 25 fractions, followed by HDR brachytherapy. Brachytherapy prescription regimens were 22 Gy in 4 fractions (3 cases), 28 Gy in 4 fractions (36 cases), and 34 Gy in 4 fractions (1 case). Ten patients were treated with Venezia applicator (inter-uterine tandem and ovoid rings), with multi-channel vaginal caps and ProGuide 294 mm sharp plastic needles with outer diameter of 2 mm. Ten patients were treated with Venezia applicator and needles and ten patients were treated with Venezia applicator only. Ten patients treated with tandem and split-ring (TSR) applicator (Eckert & Ziegler BEBIG, Germany; Fig. 1A, B) were included and compared with plans of only using Venezia applicator. All patients underwent computed tomography (CT) scan with 2 mm slice thickness at 140 kVp, with metal artefact reduction feature enabled. All the brachytherapy plans were generated in Oncentra version 3.5 (Elekta, Sweden) treatment planning system (TPS). Both EBRT and brachytherapy doses were computed as biologically equivalent doses in 2 Gy fractions (EQD2) for a dose composite. A uniform 45 Gy EBRT was used, and the composited EQD2 of EBRT and brachytherapy for target and OAR followed the GEC-ESTRO and the ABS recommendations [1, 13].
Applicator volume removal
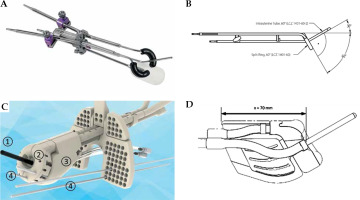
The Venezia (VZ) applicator consists of an intra-uterine (IU) tandem with lengths ranging from 30 mm to 70 mm and angles of 15º to 30º, with two lunar-shaped ovoid rings of 22 mm and 26 mm, respectively (Fig. 1C). Vaginal caps are additional components for vaginal disease irradiation. The caps are of around 60 mm in length, and they have three different diameters to match lunar ovoid rings (Fig. 1D). The IU TSR applicator is made of titanium (ρ = 4.50 g/cm3), and build-up caps for the rings are made of polyphenylsulfone (PPSU, ρ = 1.31 g/cm3) [11, 18].
The plan and dose DICOM files were then transferred from TPS to MIM Maestro (version 6.8, MIM Inc., USA) for applicator contouring, applicator removal, and dosimetric comparisons. Major materials for the applicator and vaginal caps are made of PPSU, epoxy (ρ = 1.25 g/cm3), and glass fiber (ρ = 2.14 g/cm3). Minimal Hounsfield unit (HU) value of 200 was used for applicator contouring.
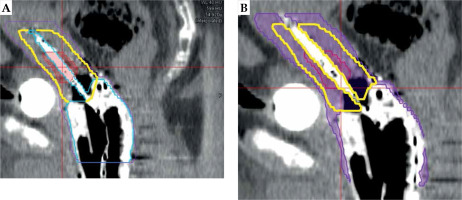
The applicator was contoured where there was target contour involvement. Therefore, the inferior contour margins for the Venezia applicator and needles were at the same level where the IR-CTV contour ended (Fig. 2A). All the needles were contoured with a fixed 2 mm diameter. Then, the contoured Venezia applicator and needles were subtracted from the target contours to generate new target contours gross tumor volume (GTV)sub, HR-CTVsub, and IR-CTVs1ub, respectively (Fig. 2B).
Dosimetric evaluation
In this study, a uniform 45 Gy of EBRT was used for EQD2 dose composite. Both EBRT and brachytherapy doses were calculated in EQD2 using a linear quadratic (LQ) model with α/β ratio of 3 for normal tissues and α/β ratio of 10 for tumor (Eq. 1 and 2).
In equation 1 and 2, n is the number of fractions, and it was unity in this study, since each fraction was considered a new plan; d is the fractional dose. DVH parameters of composited EBRT and brachytherapy were achieved using full parameter addition, where EQD2 doses were numerically added.
Dosimetric parameters, such as D90%, D50%, V100%, V150%, and V200% were evaluated. Plans with and without vaginal caps were further divided into two groups for IR-CTV DVH parameters comparison. TCP was calculated based on linear quadratic model [19, 20], where biologically equivalent dose (BED) was applied.
Results
The volumes of targets and VZ applicators contoured are listed in Table 1. The average contoured VZ applicator volume was 30.9 ±14.8 cm3. The average volume changes in GTV, HR-CTV, and IR-CTV after VZ applicator removal were 1.5 ±1.5 cm3, 15.8 ±6.4 cm3 and 33.8 ±15.1 cm3, respectively.
Table 1
Volumes of target and applicator contours
Table 2 demonstrate DVH parameter changes after VZ applicator removal. Changes in D90% of GTV, HR-CTV, and IR-CTV were 2.1% ±0.8%, 4.6% ±2.8%, and 7.7% ±5.2%, respectively. Among all the cases, only one case had D90% of EQD2 dose composite reduced to less than 80 Gyα/β = 10 (from 83.9 Gyα/β = 10 to 77.7 Gyα/β = 10 after applicator removal), which was the hard constraint for HR-CTV recommended by the ABS [1]. The VZ applicator exclusion resulted in significant changes (p < 0.05) in small volume parameters (e.g., D50%) and high-dose parameters (e.g., V150%, V200%) for HR-CTV and IR-CTV (Table 2). In addition, IR-CTV had the largest changes in D90%, D50%, V100%, and V150%, while HR-CTV presented the largest change in V200% after applicator volume removal. One plan with vaginal caps and IR-CTV was limited around the caps and demonstrated the maximal change. In Figure 3, the group with vaginal caps showed higher changes in D90%, D50%, V100%, and V150% compared to the group without caps (Table 3). Moreover, no significant changes were found in TCP after applicator removal.
Fig. 3
Comparisons of changes in DVH parameters after applicator removal between groups with and without vaginal caps. “GTV cap”, “HR-CTV cap”, “IR-CTV cap” are GTV, HR-CTV, IR-CTV of cases treated with vaginal caps, respectively
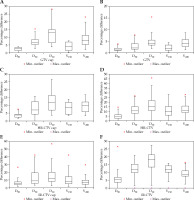
Table 2
Changes in DVH parameters and TCP of GTV, HR-CTV, and IR-CTV after Venezia applicator contour exclusion
Table 3
Changes in DVH parameters and TCP of GTV, HR-CTV, and IR-CTV after tandem and split-ring contour exclusion
The average contoured TSR applicator volume was 6.9 ±4.2 cm3. Changes in the target DVH parameter after TSR exclusion were mostly less than 5%, except for IR-CTV V200% (maximum, 6.1%; mean, 3.7% ±2.2%) and IR-CTV D50% (maximum, 6.5%; mean, 3.5% ±2.3%). There were no significant changes in the target DVH parameters after TSR applicator contour exclusion.
Discussion
Although the metal artefact reduction feature was applied, contouring accuracy was still affected by the metal artifacts caused by radiopaque CT markers and the volume averaging effect. This agrees with the fact that artefacts were more obvious in plastic needles with radiopaque CT markers or stylets inserted. The HU-based contouring method can reduce contouring uncertainty resulted from image quality among different observers. Oncentra TPS is capable of importing three-dimensional applicator model from the library, and the reconstructing it accurate in the patient CT scan by registering specific anchor points [23]. However, current treatment planning system cannot generate applicator contours automatically. Therefore, a future solution would implement the feature of automatic applicator contour generation in TPS and exclusion from target for dosimetric evaluation.
Desbien et al. reported that, after removing Syed-Neblett template, the average changes in CTV D90%, V100%, V150%, and V200% were 2.40%, 0.70%, 3.45%, and 1.18%, respectively [5]. Different types of applicators may have different target volume involvements and dosimetric impact. In this study, the mean difference in HR-CTV D90%, V100%, V150%, and V200% were 4.6%, 1.2%, 8.9%, and 11.3%, respectively. This study demonstrated larger differences in V150% and V200%, which indicates that more applicator volumes were involved with high doses. Applicator exclusion caused large changes in D50%, which agrees with the statement that small volume DVH parameters are more affected when more applicator volumes are included in target contours [2]. Although D50% is of less clinical importance, it is mainly used in studies for dosimetric comparisons between plans or different treatment modalities [23-25]. Clinical implications of these small volume DVH parameters still require further investigation.
Plans with vaginal caps demonstrated higher changes in all DVH parameters for IR-CTV after applicator volume removal (Table 4). All the plans followed soft constraint of at least 65 Gy in EQD2 (EBRT and brachytherapy) for IR-CTV D98% according to EMBRACE II. This goal could be achieved with 90% to 100% of the prescription dose of brachytherapy. Therefore, compared to tandem and ovoid rings, vaginal caps were not used for delivering high doses (e.g., 150-200% of prescription dose) to the target (Fig. 4). If IR-CTV is mainly around vaginal caps then applicator removal will have more impact on DVH parameters changes since the dose is more restricted within vaginal caps (Fig. 5). One weakness of this study is the limited number of cases treated with VZ applicator with vaginal caps. A further study on VZ applicator with vaginal caps among multiple institutions may provide more clinical supports on the necessity of VZ applicator exclusion.
Fig. 4
Dose distributions at central tandem, 1) where 200% isodose line was outside of applicator and vaginal caps; 2) where 150% and 200% of prescription dose (PD) were within applicator. Green: 100% PD, orange: 150% PD, crimson: 200% PD, pink: HR-CTV, purple: IR-CTV
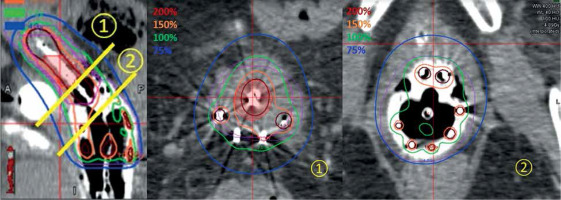
Fig. 5
Illustration of one plan, where IR-CTV was mainly around vaginal caps. Green: 100% of prescription dose (PD), orange: 150% PD, crimson: 200% PD, pink: HR-CTV, purple: IR-CTV
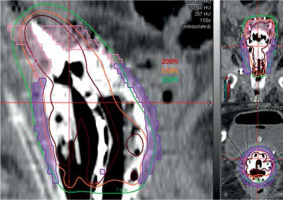
Table 4
Comparison of changes in DVH parameters of IR-CTV between cases using Venezia applicator with and without vaginal caps
Conclusions
The impact of applicator contour exclusion on target DVH parameters was evaluated for Venezia and tandem and split-ring applicators used in our institution. The Venezia applicator with vaginal caps has significant impact on small volume and high-dose DVH parameters of the target. When the Venezia applicator is used, applicator contour exclusion is recommended for more accurate dosimetric evaluation.