INTRODUCTION
Ashwagandha (Withania somnifera) is a plant whose roots are used in traditional Eastern medicine to increase resistance to physical and mental stress. The active constituents of ashwagandha extracts that may be responsible for the health properties are believed to include steroidal lactones (withanolides) and flavonoids, as well as saponins and sterane derivatives. A number of studies have reported the potential of these substances to regulate adrenal functions, among others, as well as effects on haematological indices [1]. An increase in blood haemoglobin (Hb) concentration and red blood cell count (RBC) have been pointed to as a key mechanism to explain the increase in maximal oxygen uptake (
There are several research papers in the available scientific literature on the effects of ashwagandha supplementation on exercise performance, conducted mainly in Indian population. The results of meta-analyses based on those studies indicated an improvement in aerobic capacity as a result of ashwagandha administration, both in untrained and trained individuals [1, 3]. However, it should be noted that in most of the papers included in the meta-analysis, the assessed index of aerobic capacity,
HIIT usually refers to “repeated series of exercises that occur at power output or speed in the high-intensity zone that takes place between the second ventilatory threshold and
Notably, the use of a rowing ergometer in HIIT training allows for the involvement of almost all muscle parts (both lower and upper body) and also the incorporation of resistance exercise elements [9]. Simulated rowing is therefore recommended as an effective way to achieve optimal health benefits and prevent chronic diseases [10]. At the same time, training on a rowing ergometer is safe for subjects (low injury rate) and is widely practiced among both young and elderly people [9].
In the study, a mobile measuring device that uses near-infrared spectroscopy (NIRS) to assess the degree of myoglobin oxygenation in the muscle cytoplasm and haemoglobin in the blood vessels of the muscle microcirculation (muscle oxygen saturation, SmO2) was used to assess the body’s exercise response [11]. In contrast to
In addition, modern oximeters make it possible to perform tests during competitive efforts under natural conditions in various sports [17, 18], and SmO2 measurements can also be helpful for determining training load (including exercise intensity, resting rate as well as training duration) [19–21]. Devices using NIRS technology most often monitor a variety of indicators, but in studies of athletes, muscle tissue oxygenation (SmO2) and total haemoglobin (tHb) are mainly analyzed. The SmO2 value reflects changes in muscle oxygen delivery and consumption, expressed as the ratio of oxygenated haemoglobin (oxyhemoglobin, HbO2) and myoglobin (oxymyoglobin, MbO2) to total haemoglobin. The tHb index is used to assess local blood volume and is not related to exercise intensity [22]. Given the advantages (non-invasive, lightweight, portable, waterproof and suitable for outdoor training), NIRS technology provides important information about muscle response to exercise as well as muscle recovery and, in view of this, was used in our research.
In addition, the authors did not encounter an article in the literature on the effect of ashwagandha administration on the local capacity of muscles to utilize oxygen. For this reason, a study was undertaken to characterize changes in aerobic capacity, muscle oxygenation and haematological parameters in the blood under the influence of 8-week HIIT in healthy male non-athletes.
In most studies evaluating the effects of ashwagandha supplementation on health parameters, the dose used was 300 mg of the extract per day, which is the dose recommended for people with sedentary lifestyles. For physically active people/athletes, a dose of 600 mg per day is recommended and this was the dose used in most studies evaluating the effects of ashwagandha on physical performance [1]. Although there are studies in the scientific literature reporting higher doses (750 or 1000 mg per day) [1], in the current study we chose a dose of 600 mg per day, taking into account the safety of administration supported by the results of most studies [1, 3], the recommendations of the supplement manufacturer and in accordance with the national guidelines (Dietary Supplement Panel Resolution) for the maximum recommended daily intake of the product.
MATERIALS AND METHODS
Participants
The study included healthy male participants. Exclusion criteria were: practicing high-performance sports, the use of tobacco products, alcohol consumption, taking any medications that increase physical performance, as well as drugs/medications that are sedative, antianxiety or sleep-inducing; orthopedic injury or past surgery within the last 6 months; chronic diseases; known intolerance of herbal supplements of similar composition; taking any herbal preparations or supplements containing antioxidant and anti-inflammatory substances within the last 3 months. The participants were recruited from the students of Faculty of Physical Education and Health in Biała Podlaska.
Before the experiment, participants were randomly assigned, in a double-blind fashion, to two groups. Initially, 41 students met the inclusion criteria and were randomly assigned to either the ashwagandha (n = 20) or placebo (n = 21) group. The R programme was used for the randomisation procedure [23]. Three participants (two from the ashwagandha group and one from the placebo group) dropped out of the study for personal reasons. Due to technical problems with measuring muscle oxygenation during the maximal graded exercise test, five students were excluded from the analysis due to incomplete data. Finally, 33 students (17 from the ashwagandha group and 16 from the placebo group) were included in the statistical analysis in this manuscript.
The study was conducted following the principles of the Declaration of Helsinki. All participants gave their consent to participate in the study and the research protocol was approved by the Local Ethics Committee of the Józef Piłsudski University of Physical Education in Warsaw (SKE 01-43/2022). Students were asked to refrain from modification of their diet during the study period.
Supplementation
Two capsules of ashwagandha extract were administered daily (2 × 300 mg per day) for a period of 8 weeks. One capsule contained 300 mg of ashwagandha root extract, KSM-66, standardized to a 5 % concentration of withanolides as measured by HPLC.
Placebo capsules were administered in the same form as ashwagandha supplement. Both the ashwagandha and placebo capsules (gelatin) were identical in appearance (size, shape, and color), and they both were manufactured by the same producer. The placebo capsules contained microcrystalline cellulose as an inert filler, magnesium salts of fatty acids, silicon dioxide (stored in bottles, which previously contained ashwaganda extract, so that the odor of ashwagandha permeated to the placebo capsules). The subjects were instructed to take the capsules twice a day after meals (breakfast and dinner). The capsules were supplied to the students weekly in dark nontransparent bottles. The participants (from both placebo and ashwagandha group) declared good tolerability of capsules, with no adverse events.
Blood sampling and hematological analyses
Blood samples from the ulnar vein were obtained in the morning (at 7:00 a.m.), after an overnight fast, prior to (pre) and after (post) an 8-week HIIT and supplementation (in resting condition; at least 48 hours after the last training session). The blood samples were collected to a tube (2 ml) with anticoagulant (EDTA) to hematological measurements. Hematological parameters were assessed immediately after blood collection, using an automated method at a local commercial diagnostic laboratory (hematology analyzer; BIOMAXIMA BM HEM 5 TS; Poland). These parameters included hemoglobin (Hb), hematocrit (Ht), red blood cells (RBC), leukocyte (LEU) and subset counts: lymphocytes (LYMPH), monocytes (MONO), total granulocytes (GR), eosinophils (EOS), basophils (BASO), as well as platelets (PLT).
Exercise performance and HIIT program
Before and after the training period (at least 48 hours after the last training session), the students completed an incremental rowing ergometer test (Concept 2 rowing ergometer) to volitional fatigue (i.e., with a gradual increase in intensity until the subjects had to stop due to exhaustion, GXT Test). The damper was set to 4–6. The test consisted of several 3-minute trials with 1-minute passive rest periods between trials (for collection of capillary blood samples). The power used in the first trial was 100 W, which increased by 30 W in each subsequent trial.
During the test, heart rate (HR) was continuously recorded using the H9 Heart Rate Sensor (Polar Electro Oy, Finland). Gas exchange was measured during each testing session using wearable and wireless breath-by-breath pulmonary gas analyzer (MetaLyzer 3B, Cortex Biophysik GmbH, Germany). Lactate concentration (LA) was measured in capillary blood, collected from the fingertip before the GXT, immediately after the subjects completed each 3-minute trial and 3 minutes after the entire test was performed, using the Super GL2 device (Dr Müller, Germany).
The following physiological parameters, as recognized indicators of aerobic exercise performance, was used to assess the impact of training on the physical condition of the subjects: 1) maximal oxygen uptake (
Eight-week HIIT was performed on a Concept 2 rowing ergometer (Morrisville, NC) under the supervision of qualified instructors. The initial incremental rowing ergometer test to volitional fatigue (performed prior to the exercise program) was preceded by 2–3 introductory sessions aimed at developing proper rowing technique.
The indexed training consisted of 3 sessions per week, with at least 1 day of rest between sessions. During the first two weeks (weeks 1–2), the training was designed to prepare participants for high-intensity efforts, so the load was 75–80% of the individual’s HRmax, measured during an incremental rowing test (5 sets of 1.5 minutes). The HIIT training programme in the following weeks followed the pattern: 5 sets of 1.5 minutes at 85% MAP (weeks 3–4), 6 sets of 1.5 minutes at 90% MAP (weeks 5–6), 7 sets of 1.5 minutes at 95% MAP (weeks 7–8) (Table 1). In all workouts (weeks 1–8), the rest intervals between sets were equal to the duration of the exercise (1:1) with 70 W power output. Each workout was preceded by a warm-up and ended with a cooling down. The warm-up consisted of 5-min continuous rowing at an intensity equivalent to a HR of 130–140 beats/min, while the cooling down involved rowing for about 3 minutes at low power (about 50 W). The HIIT training program was developed based on the work of Driller et al. [24] with modifications.
Anaerobic threshold and Maximal Aerobic Power
Anaerobic threshold (4 mmol, PAT4) were determined based on the application described by Newell et al. [25]. Maximal aerobic power (MAP) was calculated as a proportion of the time and a power of the last executed bout in the GXT [26]. The equation for calculation is presented below:
MAP – maximal aerobic power; MLFE – power of the last fully executed step; TLE – time executed in the final step; TSt – time of the last step; ΔP – increase in power between last two steps.
Maximal Oxygen Consumption
Maximal oxygen uptake (
Measurements of muscle oxygen saturation
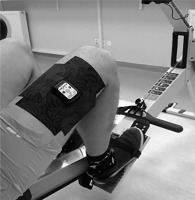
During GXT test, a NIRS device (Moxy Monitor; Fortiori Design LLC, Hutchinson, MN, USA) was placed on the vastus lateralis (VL) muscle that is active during rowing. Figure 1 shows the placement of the NIRS monitor.
The Moxy monitor is a continuous wave near-infrared spectroscopy monitor. It uses a new type of algorithm that is based on Monte Carlo modeling. The system uses 4 wavelengths at 680, 720, 760, and 800 nm. It has 2 emitters to detector spacings of 12.5 and 25 mm. The device was placed approximately 15 ± 2 cm above the proximal border of the patella on the vastus lateralis muscle belly and was fixed to the right limb with a dark 7.5 cm dynamic tape by the same person. In students, skinfold was measured at the site where the Moxy Monitor was placed. It should be emphasized that the NIRS signal is affected by the thickness of subcutaneous adipose tissue thickness (ATT), e.g., 5 mm thickness reduces the penetration of infrared light by about 20% [27, 28].
The SmO2 was recorded during exercise and recovery. SmO2 reflects the dynamic balance between oxygen (O2) consumption and supply [29]. The differences in SmO2 (Δ SmO2) between maximal and minimal exercise levels were also calculated. The reoxygenation rate after GXT test were evaluated as the 50% time required for SmO2 recovery (SmO2 Recovery Half Time, SmO2HTR) [30, 31], Fig. 2.
FIG. 2
Method of evaluating the time for 50% recovery muscle oxygen saturation (SmO2 Recovery Half Time, SmO2HTR) after the maximal graded exercise test (GXT) for one of the male students (compiled according to Nagasawa 2013 [30])
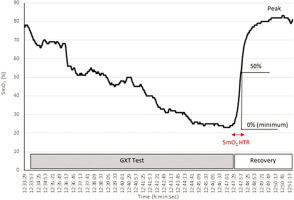
The SmO2 data values for further calculations were averaged from measurements made during 2 s at exercise minimum (SmO2 Min) and recovery maximum (SmO2 Max). The mean SmO2 for 2 seconds immediately after the completion of the exercise was defined as 0% and the maximum SmO2 in the first 3 min of the recovery phase after completion of exercise was defined as 100%. The SmO2 Δ Time was then defined as the time from the completion of exercise to the time to reach 50% SmO2Max. SmO2 Reoxy Rate was calculated according to the following formula (18):
SmO2 threshold values were determined by averaging 30-s intervals for the respective exercise loads.
Statistical analysis
Statistical analysis was conducted with Statistica version 13.3 (Stat-Soft, Krakow, Poland). Normality assumptions were checked on all variables using Shapiro–Wilk test and visual inspection (quantile distribution plots). Also, homogeneity of variance was checked with Levene’s test. All values were reported as mean ± standard deviation (SD). The level of statistical significance was set at p < 0.05. Statistical significance of intergroup (ashwagandha vs. placebo) differences in anthropometric characteristics (age, height, etc.) were verified with unpaired Student t-test. The two-way ANOVA for repeated measurements: 2 groups (placebo, ashwagandha) × 2 time points (pre-, post-) was applied for comparison of muscle oxygenation and the performance results obtained in the maximal graded exercise test. For detailed comparisons (between and within groups) the post-hoc Tukey´s test for unequal samples was utilised. Furthermore, the effect size for the ANOVA was estimated (eta squared; η2). The same statistical methods were used for haematological blood indices, however, after logarithmic transformation (natural logarithm). Sample size was calculated using GPower version 3.1 [32]. With the assumptions: effect size f = 0.25, α = 0.05, power 0.8, required sample size amounted 34 for two-way ANOVA with repeated measurements (two time points). The power for the group of 33 participants in our study taken for analysis was 0.79.
RESULTS
The characteristics of the subjects are shown in Table 2. In terms of the anthropometric indices analyzed, as well as maximum oxygen uptake at the beginning of the study, there were no statistically significant differences between the study groups (P > 0.05).
TABLE 2
Basic characteristics of the examined group of students (mean ± SD).
The mean values of selected indices of exercise capacity are shown in Table 3. No effects of group or time × group interaction were shown in any of the variables analyzed. In turn, main effect of time was found for test time (P = 0.00001; η2 = 0.77), MAP and PAT4 (in both absolute and relative values; P = 0.00001; with η2 amounted 0.60–0.78), as well as in
TABLE 3
Values of selected indices recorded during the maximal graded exercise test performed before (pre) and after (post) 8-week HIIT training combined with ashwagandha (n = 17) or placebo (n = 16) supplementation.
| Variable/Group | Time | Ashwagandha (n = 17) | Placebo (n = 16) | Main effects: P-Values (η2, Effect Size) | ||
|---|---|---|---|---|---|---|
| Time | Group | Time × Group | ||||
| Test time (min:s) | Pre | 15:14 ± 3:31 | 15:39 ± 3:33 | 0.00001 | 0.60 | 0.47 |
| Post | 17:27 ± 2:42*** | 18:13 ± 3:31*** | (0.77) | (0.009) | (0.02) | |
| MAP (W) | Pre | 226 ± 36 | 230 ± 51 | 0.00001 | 0.64 | 0.38 |
| Post | 252 ± 32*** | 261 ± 49*** | (0.78) | (0.007) | (0.02) | |
| MAP (W/kg) | Pre | 2.81 ± 0.49 | 2.90 ± 0.46 | 0.00001 | 0.41 | 0.28 |
| Post | 3.13 ± 0.46*** | 3.29 ± 0.41*** | (0.77) | (0.02) | (0.04) | |
| Pre | 3.65 ± 0.58 | 3.66 ± 0.61 | 0.019 | 0.84 | 0.66 | |
| Post | 3.77 ± 0.41 | 3.84 ± 0.68 | (0.16) | (0.001) | (0.006) | |
| Pre | 46.1 ± 6.7 | 46.9 ± 4.5 | 0.15 | 0.54 | 0.55 | |
| Post | 46.7 ± 6.2 | 48.3 ± 5.5 | (0.06) | (0.01) | (0.01) | |
| LAPEAK (mmol/l) | Pre | 12.3 ± 2.6 | 12.1 ± 2.8 | 0.43 | 0.82 | 0.31 |
| Post | 12.2 ± 1.6 | 12.8 ± 2.7 | (0.02) | (0.002) | (0.03) | |
| PAT4 (W) | Pre | 151 ± 38 | 150 ± 44 | 0.00001 | 0.87 | 0.52 |
| Post | 176 ± 35*** | 181 ± 41*** | (0.60) | (0.0008) | (0.01) | |
| PAT4 (W/kg) | Pre | 1.86 ± 0.47 | 1.89 ± 0.42 | 0.00001 | 0.67 | 0.44 |
| Post | 2.17 ± 0.42*** | 2.27 ± 0.39*** | (0.61) | (0.006) | (0.02) | |
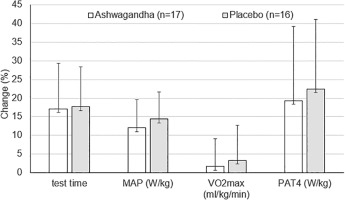
FIG. 3
Percentage changes in test time, maximal aerobic power (MAP) (W/kg), maximal oxygen uptake (VO2max) (ml/kg/min) and power at anaerobic threshold (PAT4) (W/kg) after 8-week HIIT training combined with ashwagandha supplementation or placebo. Values are mean ± SD
No inter-group differences were found at p < 0.05 (unpaired Student t-test).
Data on muscle oxygenation parameters recorded during the maximal graded exercise test are shown in Table 4. Statistical analysis revealed a significant time and group interaction for SmO2Min (P = 0.026; η2 = 0.15) and ΔSmO2 (P = 0.011; η2 = 0.19), with post-hoc analyses revealed significant pre-post differences in the placebo group (i.e. a decrease in SmO2Min, P < 0.05; and an increase in ΔSmO2, P < 0.01), whereas no changes were found in the ashwagandha group (P > 0.05). In turn, post-hoc analysis showed no significant intergroup differences in the above-mentioned parameters (SmO2Min and ΔSmO2), for both pre (P > 0.05) and post intervention (P > 0.05). Moreover, a main time effect was found for SmO2Max (P = 0.016; η2 = 0.17), and ΔSmO2 (P = 0.0039; η2 = 0.24).
TABLE 4
Values of selected indices of muscle oxygenation recorded during the maximal graded exercise test performed before (pre) and after (post) 8-week HIIT training combined with ashwagandha (n = 17) or placebo (n = 16) supplementation.
[i] Values are mean ± SD. *, **– significant difference (p < 0.05 and p < 0.01, respectively) between pre and post values within the same group (ashwagandha or placebo). Abbreviations: SmO2 Max – maximum muscle oxygenation; SmO2 Min – minimum muscle oxygenation; ΔS mO2 (%) – SmO2 Max minus SmO2 Min; SmO2HTR(s) – the time from the completion of exercise to reach 50% SmO2Max; SmO2 ReoxyRate(%/s) – recovery rate of muscle oxygenation; η2 – eta squared (Effect Size).
Mean values of haematological parameters are shown in table 5. No significant main effects (i.e. time, group) and time and group interaction were found for most of these parameters (except for EOS (10 × 3/µl) and MONO (%)). A main effect of time (P = 0.03) was found for EOS (in absolute values), with post-hoc analysis showing only an increasing trend for the placebo group (P = 0.1), but not for the ashwagandha group (P = 0.8). Regarding MONO in relative terms (%), despite a significant main effect of time (P = 0.04), no significant post-hoc analyses were found in both ashwagandha (P = 0.7) and placebo (P = 0.3) groups. Furthermore, in both groups and at both measurement periods (before and after the 8-week study period), the mean values of all haematological parameters were within the reference range for the study population.
TABLE 5
Haematological blood indices of students before (pre) and after (post) 8-week high intense interval training (HIIT) combined with ashwagandha supplementation (n = 17) or placebo (n = 16).
[i] Values are mean ± SD. No significant differences were seen between term 1 and term 2 (within the same group) or between groups (within the same term: 1 or 2) (P > 0.05). Abbreviations: WBC: white blood cell count; LYMPH: lymphocytes; MONO: monocytes; GR: granulocytes; EO: eosinophils; BASO: basophils; RBC: red blood cell count; Hb: haemoglobin; Hct: hematocrit; PLT: platelet count.
DISCUSSION
The main finding of our study is that ashwagandha supplementation does not seem to affect training-induced improvements in aerobic capacity in healthy men. Despite significant increases in aerobic performance indices (test time, MAP and PAT4 in absolute and relative values) under 8-week HIIT, there was no greater improvements in the ashwagandha group compared to the placebo group (Table 3). The observed changes in the aforementioned indices of aerobic capacity, such as post-training increases in test time (by 17.1 ± 12.3 and 17.7 ± 10.7%), as well as MAP in relative units (by 12.1 ± 7.6 and 14.4 ± 7.2%) and PAT4 in relative units (by 19.3 ± 19.9 and 22.5 ± 18.6%) in the ashwagandha and placebo groups, respectively, indicate that HIIT is highly effective in improving aerobic capacity using the rowing ergometer (Fig. 3).
Our finding did not confirm the previous results of a meta-analysis based on five studies conducted in the Indian population indicating improvements in aerobic capacity after 4–12 weeks of ashwagandha supplementation (at a dose of 300–1000 mg per day), both in untrained and trained individuals [1, 3]. However, it should be mentioned that two of these five papers [2, 33] used indirect methods, including the Cooper test, to calculate
In order to test the potential mechanisms responsible for the improvement in aerobic capacity as a result of ashwagandha supplementation, as described in previous papers [1, 3], muscle oxygenation parameters were monitored during GXT in our study. In the available literature there are relatively few reports evaluating the effect of training on the characteristics of changes in muscle oxygenation indices [18, 20, 38], and the observation above is particularly applicable to HIIT [19]. The last cited study documented the effects of four various types of HIIT on changes in muscle oxygenation indices in sprint canoe-kayak athletes. Interestingly in our own research, 8-week HIIT alone appears to affect changes in SmO2 Max and ΔSmO2 (significant main effect of time), as well as SmO2Min (a tendency toward a time effect). However, contrary to expectations, significant changes in ΔSmO2 and SmO2Min after the 8-week study period were observed in the placebo group, but not in the ashwagandha group, with significant interaction of time and group for SmO2Min and ΔSmO2 (Table 4). Namely, only in the placebo group was there an improvement in the degree of oxygen utilization by working muscles (ΔSmO2) after 8-week training, which was mainly due to a decrease in minimum exercise SmO2 (Table 4). Generally, the lowest muscle O2 levels (SmO2Min) is considered to be indirectly related to exercise intensity (muscle O2 consumption) (39, 40). Thus, lower SmO2Min in the placebo group after training than before training may have been related to higher intensity of the test performed post-training (higher O2 consumption), as compared to pre-training. On the other hand, our blood lactate results do not seem to support this hypothesis, as there were no significant differences in blood LApeak (as a marker of exercise intensity). However, it should be noted that in the placebo group there was a slight trend towards higher LApeak after HIIT training (12.8 ± 2.7 mmol/l) compared to baseline (12.1 ± 2.8 mmol/l), Table 3. Such a response was not observed in the ashwagandha group (12.3 ± 2.6 vs 12.2 ± 1.6 mmol/l, respectively). On the other hand, the lack of any significant differences in LApeak cannot exclude the possibility that, after 8-week training (compared to the pre-training condition), individuals in the placebo group may have performed the test at a higher intensity (with more intensified anaerobic glycolysis and muscle lactate production). Undoubtedly, additional information could be provided by the determination of blood acid-base balance indices, which can be considered as some limitations of this study. On the other hand, it is difficult to explain why these muscle oxygenation changes apply to the placebo but not to ashwagandha group, even suggesting some blunting effect of ashwagandha on training-induced improvements in muscle oxygenation. These issues need to be explained in further study.
Finally, although increased muscle oxygen utilization was observed only in the placebo group, improvements in maximal oxygen uptake in absolute terms occurred in both groups (by 4.9 ± 15.3% and 4.8 ± 6.8% in the ashwagandha and placebo group, respectively), Table 3. However, in relative units,
In addition, the recovery rate indices (SmO2HTR and SmO2 ReoxyRate) also showed no significant changes in the two groups after training, Table 4. Importantly, in our previous study, the rate of SmO2 reoxygenation showed a significant correlation with
In contrast to the cited studies, in both groups of the current study, the 8-week training period did not increase the rate of SmO2 recovery. It can be speculated that this was too short a training period to affect, in addition to improvements in test duration, MAP and PAT4, other adaptive changes typically associated with endurance training. These included an improvement in muscle fiber capillarization, an increase in the total number of mitochondria and mitochondrial size, as well as an increase in oxidative enzyme activity and myoglobin content [42]. Indeed, the aforementioned morphological and metabolic changes in skeletal muscle are associated with improved recovery rates, and the relatively short training period in our study was likely insufficient to induce them.
The conclusion that there is no beneficial effect of ashwagandha administration on aerobic exercise capacity is further strengthened by the observed lack of differences between the study groups in terms of anaerobic threshold power, Table 3. In contrast to maximal oxygen uptake, exercise indices at the anaerobic threshold level are generally considered more sensitive to training (compare Fig. 3). However, again, no more favorable threshold power results were obtained in the ashwagandha group, as compared to the placebo group.
On the other hand, as already mentioned, potential effect of ashwagandha supplementation on the parameters of muscle oxygenation and their changes induced by training need to further explanation, as the results obtained are difficult to compare due to the lack of studies with ashwagandha supplementation using the discussed methods of analyzing muscle oxygenation under exercise conditions.
The possible beneficial effects of ashwagandha on aerobic capacity may be mediated through red blood cell indices (RBC, Hb, Ht) as reported in a recent review study [43]. However, these results refer to studies conducted without a placebo group [44] or from studies whose results need to be confirmed due to the lack of significant intergroup differences [2]. Our double-blind placebo-controlled study did not confirm the above-mentioned observations, given the non-significant time and group interaction in haematological indices (Table 5). As mentioned above, it cannot be excluded that higher dose of ashwagandha might be needed to exert beneficial effects in healthy young men. In the study of Raut et al. [44], increasing daily dosage of ashwagandha extract every 10 days was used (750 mg/day × 10 days, 1000 mg/day × 10 days, 1250 mg/day × 10 days) in healthy volunteers, but without any training program. On the other hand, the supplementation regimen used in the study by Malik et al [2] in young hockey players was similar to that in our study (500 mg daily for 8 weeks). Our findings regarding the lack of effect of ashwagandha on haematological indices are consistent with the results reported by Lopresti et al. [45], although his study involved the intake of ashwagandha extract (at the same dose as in our study, i.e. 2 × 300 mg daily for 8 weeks) in mildly anxious, healthy overweight men aged 40–70 years. Also in our previous study on professional wrestlers [46], we found no effect of ashwagandha supplementation (2 × 300 mg/day for 8 weeks) on haematological indices. Moreover, no significant changes in haematological indices were found in the study of Ziegenfuss et al. [47] after 12-week ashwagandha supplementation (500 mg dose daily) in recreationally active young underwent strength training. Precisely, beneficial effects was seen rather in placebo instead in ashwagandha group, i.e. the increase in RBC, Hb and Ht [47]. Therefore, further research is needed to resolve these discrepancies in results regarding the effects of ashwagandha on aerobic capacity and the mechanisms responsible for these effects.
CONCLUSIONS
In conclusion, in healthy men subjected to an 8-week HIIT, ashwagandha supplementation does not appear to affect haematological status or offer additional benefits in aerobic capacity over those observed under training. Moreover, the inconclusive effect of ashwagandha on training-induced changes in muscle oxygenation needs to be verified in further studies.