Purpose
Oral tongue cancer is responsible for approximately 2% of global cancer-related mortality annually [1]. Among oral cavity cancers, tongue squamous cell carcinoma (TSCC) is the most frequent tumor, accounting for a substantial proportion of cases, with a prevalence ranging from 22% to 49% [2]. The incidence of TSCC varies geographically, with higher rates observed in regions characterized by high tobacco use, alcohol consumption, and poor oral hygiene [3-5]. These risk factors contribute to genetic mutations and cellular abnormalities, ultimately leading to the development of TSCC [6].
The treatment of TSCC typically involves a multidisciplinary approach. Early stage TSCC, classified as stage I-II with a tumor size of less than 4 cm and no lymph node or distant metastasis, carries a relatively good prognosis compared with locally advanced or metastatic tumors [7, 8]. In early stages of TSCC, the primary treatment modalities involve surgical intervention and radiotherapy, which may be utilized individually or in combination [9-11]. The selection of adjuvant treatment techniques is based on various factors, such as close or positive margins, perineural invasion, vascular invasion, and lymphatic invasion [12, 13]. Patients with T1-T2 tongue cancer, who undergo surgery with or without adjuvant therapy, reported a five-year survival rate of over 60% [14].
In recent years, significant advancements have been made in the adjuvant treatment after curative surgery in TSCC patients. Two prominent treatment modalities, which gained substantial attention are external beam radiotherapy (EBRT) and interstitial brachytherapy (BT) [9]. Furthermore, updated GEC-ESTRO guideline focused on the modern BT therapy as a successful treatment for function preservation and cosmetic outcomes in head and neck cancers [15]. EBRT performed by various techniques, involves using a linear accelerator to deliver high-energy radiation beams externally to the tumor site, and targeting the primary tumor bed and regional lymph nodes. In contrast, BT utilizes the placement of radioactive sources directly into the tumor bed, allowing for precise and localized radiation delivery [16, 17]. Both approaches aim to deliver targeted radiation to the tumor site to eradicate residual disease and improve patient outcomes [18]. The critical difference between these techniques lies in their radiation delivery characteristics. EBRT provides a non-invasive approach that can cover a more extensive treatment field, while interstitial BT offers a more conformal and concentrated dose distribution, but requires sufficient skills and a learning curve [19-21]. The choice of a technique depends on various factors, including tumor stage, location, surgical bed anatomy, and patient-specific considerations.
This article compared the potential impact of BT and EBRT on survival outcomes, focusing on overall survival (OS) and disease-free survival (DFS) as essential treatment goals. Understanding the nuances of these adjuvant radiotherapy techniques is crucial for making personalized treatment decisions, and optimizing the management of patients with TSCC.
Material and methods
Patients and study design
In this retrospective cohort study, the objective was to evaluate the efficacy of the type of radiotherapy in survival outcomes and local and distant metastasis of patients diagnosed with tongue squamous cell carcinoma (TSCC), who underwent curative surgery. A thorough examination of medical records from the Department of Radiation Oncology was performed. A total of 84 patients (37 females and 47 males) with pathologically confirmed TSCC, who underwent both surgery and adjuvant radiotherapy were included in the study. Data collection was conducted from 2011 to 2022 in the Department of Radiation Oncology, Cancer Institute of Iran, Tehran. Study protocol received approval from institution’s review board and ethics committee (ethics code No: IR.TUMS.IKHC.REC.1400.397). Ethical considerations were strictly followed according to the principles of the World Medical Association Declaration of Helsinki amended in October 2013 [22].
Inclusion and exclusion criteria
To ensure high level of homogeneity within the study population, the following inclusion criteria were determined: Patients who underwent surgery with adjuvant radiotherapy, and had T3 or T4 or N+ pathology post-surgery, positive close margins, excisional biopsy, evidence of lymphovascular or perineural invasion, extra-nodal lymph node involvement, involvement of tongue stratified muscles, and no detectable metastases. According to these criteria, a total of 84 patients were included for further analysis.
Pre-analysis evaluation
In order to gather the necessary clinical data for the study, all relevant information from patients’ medical records were retrieved. These involved extracting demographic details from history-taking forms, diagnostic information from reports of different modalities (i.e., CT scans, contrast-enhanced MRI, and neck ultrasonography), histopathology reports, treatment-specific data from surgery summaries, pathology reports, and radiotherapy and chemotherapy documentation. Additionally, data from follow-up visits during the 5-year observation period were collected, including recurrence rates and survival analysis. For patients with no complete follow-up or comprehensive data, telephone interviews were conducted to obtain supplementary information.
Clinical staging and treatment approaches
The 8th edition of the American Joint Committee of Cancer TNM staging system was employed to assess tumor dissemination within local and regional areas [23]. The enrolled patients were divided into early stage patients with stage I or II tumors (T1-2 N0), and locally advanced-stage patients with stage III or IV (T3-4 N any). Also, patients were categorized based on radiotherapy type, resulting in three groups: brachytherapy (BT) alone, external-beam radiotherapy (EBRT) alone, and a combination of both. At our center, patient selection for adjuvant radiotherapy depends on various challenges, and is primarily based on the expertise required for brachytherapy and the physician’s decision regarding its application. Factors influencing this decision include whether or not to avoid neck treatment, the extent of involvement at the base of the tongue, and challenges posed by reconstructive procedures in the tongue area, which can complicate brachytherapy process.
Before initiating treatment, a dental consultation was obligatory, and any necessary interventions were performed approximately 10 days before the first treatment fraction. During radiotherapy, patients underwent regular monitoring to assess any potential adverse effects. Patients undergoing BT were admitted and visited daily in our inpatient ward, while those undergoing EBRT were observed weekly in outpatient ward to determine any potential treatment-related side effects. In some instances, temporary interference was implemented at the attending physician’s discretion.
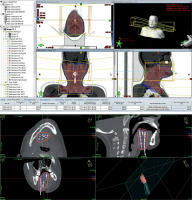
Patient immobilization, simulation, and treatment planning process strictly followed the routine protocols of our oncology department. The oncologists at our radiation center delineated the volume of clinical targets using surgery-related data from patients’ records and different modalities, such as pre-operative MRI and CT scan. Diverse dosing schedules were chosen based on specific radiation modality employed in the study. For patients undergoing interstitial BT alone, a total dose of 39 Gy was administered in 3 Gy fractions, twice a day, five days a week, with a six-hour interval between each fraction. Furthermore, for patients receiving EBRT alone, a minimum of 60 Gy was delivered in 2 Gy fractions, with treatment sessions occurring five times per week, in 6-6.5 weeks. The prescribed treatment for the combination therapy consisting of BT and EBRT was a BT dose of 15-21 Gy in 3 Gy fractions twice a day, typically followed by 44-46 Gy of EBRT administered in daily 2 Gy fractions. There was usually a two-week gap between these two treatments. For EBRT planning, photons with an energy of 6 MV were used to cover target volume with 95% isodoses. A routine beam arrangement consisted of two lateral opposed portals using wedges and shields for the oral cavity and upper neck and an en face supraclavicular with or without an opposing posterior portal for the lower neck. BT details have been described elsewhere [10]. Figure 1 illustrates the dosimetry of brachytherapy techniques.
Study endpoints and follow-up
Following the completion of radiotherapy, a structured schedule of follow-up visits was established. These visits occurred at specific intervals: one-month post-radiotherapy, every 3-4 months during the first two years, every 6 months from years 3 to 5, and annually thereafter. Patients who experienced symptoms related to their pre-cancer history were advised to consult their physician initially. Follow-up procedures included routine clinical evaluation and radiological examination (ultrasound imaging every three months and magnetic resonance imaging (MRI) every six months) for all patients. If there was a suspicion of metastasis or clinical indications suggestive of recurrence, additional imaging techniques (ultrasound, computed tomography (CT) scans, or whole-body bone scans) were conducted. In cases where cancer recurrence was suspected, verification through biopsy was sought. Patients with established local recurrence were discussed at multi-disciplinary tumor boards, and then received re-radiation, re-resection, or systemic therapy with immunotherapy and chemotherapy based on PD-L1 status.
Statistical analysis
Statistical analysis aimed to investigate the relationship between the type of radiotherapy used in patients with tongue cancer and clinical endpoints, such as loco-regional recurrence (LRR), loco-regional control (LRC), DFS, OS, and oral cavity recurrence. Our analysis comprised various parameters to demonstrate this association, including age, gender, biopsy type, tumor-related parameters (i.e., size, depth of invasion, extent, and stage), lymph node condition, necessary neck dissection, concurrent chemotherapy, radiotherapy-related parameters (i.e., dosage and treatment time), and surgery-related parameters, such as grade, type, margin involvement, closest margin, and the need for re-resection.
The evaluation of the study focused on several endpoints, including LRC, LRR, DFS, oral cavity recurrence, and OS. These endpoints were calculated from the date of last radiotherapy session until death or any type of recurrence (i.e., local, regional, or distant recurrence), or until the last follow-up of surviving patients without any events. The study aimed to compare these endpoints through different sub-groups of early stage tumors with the type of radiotherapy treatment.
Median and standard deviations (SD) were presented for continuous variables, and percentages for categorical variables. Kaplan-Meier method was used to depict graphs for comparing various radiotherapy treatment methods. Three survival outcomes, such as OS, DFS, and LR were demonstrated in graphs. Hazard ratios (HR) and 95% confidence interval (CI) were provided by Cox proportional hazards model. Statistical analysis was performed using R software (version 4.3) using survival, survminer, and forestploter packages.
Results
Patients’ characteristics
Eighty-four patients who met the inclusion criteria were covered in the final analysis, with a median follow-up duration of 70 (range, 4-132) months. There were no significant differences in the baseline characteristics of the patients among the three radiotherapy methods, except for tumor size and invasion depth. The mean ages of the patients were 52 ±15, 52 ±16, and 54 ±11 years for EBRT alone, BT monotherapy, and EBRT + BT, respectively. Slightly more than half of the patients were males (55.9%). Additional detailed characteristics of the patients with tumor stage are presented in Table 1.
Table 1
Baseline clinical and surgery-related characteristics
a,b , ab Only values without overlapping letters have significant differences when p-value in that row is < 0.05, BT – brachytherapy, EBRT – external beam radiotherapy, mm – millimeter, RT – radiotherapy, # The varying total numbers for each characteristic in Table 1 are due to missing data for certain variables among the patients
Radiotherapy-related characteristics
Assuming an α/β ratio of 10 for TSCC, the mean EQD2 total dose for patients undergoing BT monotherapy was 42.3 Gy over 8 days. For EBRT alone, a total dose of 60 Gy was administered over a median duration of 43 days. In the case of EBRT + BT, a median total dose of 19.5 Gy was administered for BT boost, and a median total dose of 46 Gy was given for EBRT, resulting in a combined median EQD2 total dose of 63.5 Gy over 34 days. The detailed information on the radiotherapy dosage in each group are provided in Table 2 and Supplementary Table 1.
Table 2
Radiotherapy-related characteristics
Outcomes
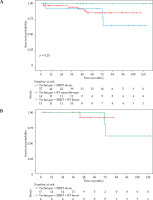
The median OS and DFS were not reached in the study’s cohort during median 70-month follow-up period. Therefore, mean values were reported. The mean OS for EBRT alone, BT monotherapy, and EBRT + BT was 90.8 (SD = 5.61), 120.8 (SD = 10.32), and 110.23 (SD = 8.36) months, respectively. Overall, no significant difference was observed in OS among the adjuvant treatments (Figure 2A). In the early stage TSCC group, the mean OS for EBRT alone and BT monotherapy was 87.8 (SD = 6.85) and 116.50 (SD = 11.411) months, respectively (HR = 3.48, 95% CI: 0.45-26.87%, p = 0.201), indicating no significant difference (Figure 2B). For locally advanced TSCC, the mean OS for EBRT alone and EBRT + BT was 72.46 (SD = 4.880) and 115.20 (SD = 6.977) months, respectively (HR = 37.57, 95% CI: 0.001-9.68%, p = 0.231) (Figure 2C).
Fig. 2
Overall survival in all patients (A) Early stage patients (B), and locally advanced patients (C)
EBRT – external beam radiotherapy, BT – brachytherapy

The mean DFS for EBRT, BT monotherapy, and EBRT + BT boost was 81.4, 116.5, and 102.8 months, respectively. Similar to OS, no significant difference was observed in DFS among the adjuvant therapies (Figure 3A). In early stage TSCC, the mean DFS for EBRT alone and BT monotherapy was 82.6 (SD = 7.18) and 116.50 (SD = 11.41) months, respectively (HR = 4.34, 95% CI: 0.57-33.11%, p = 0.120) (Figure 3B). For locally advanced TSCC cases, the mean DFS for EBRT alone and EBRT + BT was 68.1 (SD = 5.98) and 102.8 (SD = 11.06) months, respectively (HR = 1.75, 95% CI: 0.29-10.69%, p = 0.535) (Figure 3C). No significant difference was observed between the adjuvant treatments in terms of DFS.
Fig. 3
Disease-free survival in all patients (A), early stage patients (B) Locally advanced patients (C)
EBRT – external beam radiotherapy, BT – brachytherapy

Loco-regional control was only defined for locally advanced TSCC due to its rare occurrence in early stage patients that prohibited practical comparisons. The mean LRC for EBRT alone and EBRT + BT was 78.5 (SD = 3.29) and 102.8 (SD = 11.06) months, respectively (HR = 0.655, 95% CI: 0.052-8.256%, p = 0.742). No significant difference was noted between the adjuvant treatments regarding LRC (Figure 4A, B).
Fig. 4
Loco-regional control analysis in all (A) and locally advanced patients (B)
EBRT – external beam radiotherapy, BT – brachytherapy
The 5-year rates of OS, DFS, and LRC are shown in Table 3. As evident, implementation of BT was associated with higher loco-regional control, and consequently improved DFS and OS. When focusing on RT technique, although non-significant, a trend towards better outcomes was observed by applying BT as monotherapy in early stage and as a boost in locally advanced cases compared with EBRT alone. However, there were nuances in these observations. It is important to be aware that cases selected for BT, either as monotherapy or as a boost, are generally fewer and easier to access than those selected for EBRT alone. However, in the current study, the differences were non-significant based on Kaplan-Meier and log-rank tests.
Table 3
5-year rates of overall survival, disease-free survival, and loco-regional control in the study cohort based on stage
Discussion
The current investigation focused on the pivotal factors associated with radiotherapy modalities and their consequential impact on patients’ outcomes. Specifically, three critical dimensions were accurately examined: OS, DFS, and LRR among individuals with early stage and locally advanced stage TSCC, who underwent surgery followed by various radiotherapy approaches. These complex analyses provided valuable insights into the intricate relationship between therapeutic intervention and clinical trajectory.
Treatment options involve a triad of approaches, such as surgery, radiotherapy (both external and brachytherapy), and chemotherapy. Remarkably, radiotherapy yields outcomes comparable to surgical intervention for early stage lesions, even when employed as a standalone modality [24]. However, due to lower complications and shorter duration of treatment in early stage tumors, surgery is the modality of choice in oral cavity SCC [25]. In the locally advanced stage, combined modality treatment is required. Some patients with early stage disease and nearly all patients with locally advanced disease, require adjuvant radiotherapy based on high-risk features for recurrence. Radiotherapy can be done using either EBRT alone, BT alone, or a combination of both the techniques. The principle of brachytherapy, guided by the inverse square law, enables selective targeting of suspicious regions while discreetly preserving adjacent normal tissues [26]. However, there are inherent disparities between these techniques. Target delineation guidelines vary between EBRT and BT planning. In EBRT, the whole oral cavity and floor of the mouth, in conjunction with myocutaneous flaps and surgical scars, are recommended to be included as high-risk CTV regions. In contrast, in BT planning, only the tumor bed is covered, and due to sharp dose fall-off, better sparing of normal tissues can be achieved that can augment patient’s compliance. During EBRT planning, the highest dose is shown in the treatment planning system, while in BT, the lowest dose is displayed. These differences may have implications for patients’ long-term outcomes, as BT cases receive much lower high-dose volume coverage than EBRT patients [17, 27].
According to the findings of the study, TSCC has a relatively long survival regardless of post-operative radiotherapy technique used. In the present study, 70% of patients were T1-2 N0, and the 5-year OS and DFS rates were 65% and 59% for EBRT alone, 86% and 86% for BT alone, and 83% and 68% for EBRT plus BT boost, respectively. The 5-year LRC was 83% and 100% for EBRT alone and BT alone vs. 85% for EBRT plus BT boost, respectively.
Previously, we reported the outcomes of post-operative interstitial brachytherapy in oral tongue cancer. The majority of the cohort (71.7%) were patients with T1-2 N0 stage; 78% of the patients were N0, and only 8.9% were T3. The 4-year OS and DFS rates were 83% and 65%, respectively. The 4-year local and loco-regional control rates achieved remarkable levels: 89.7% and 87.2%, respectively [10]. The 4-year LRC was 81.4% vs. 90% of those receiving BT alone vs. EBRT plus BT boost, respectively. Notably, tumor dimensions, nodal status, gender, and radiation modality (specifically BT alone vs. combined BT and EBRT) demonstrated no statistically significant correlation with local control rate. Other studies also showed promising results [28, 29]. A study comparing high-dose-rate brachytherapy (HDR-BT) alone and perioperative brachytherapy (PB) in early mobile tongue cancer patients reported that PB had higher overall survival (92.3% vs. 74.7%) and disease-free survival (92.3% vs. 55.3%) at 6 years. This was due to the control of the neck with surgery and high rates of nodal failure using HDR-BT alone [30]. In another study by Takacsi-Nagy et al. in low-risk early stage oral tongue SCC following post-operative HDR-BT, the 10-year local and regional control (LC, RC), overall survival (OS), and disease-specific survival (DSS) rates were 85%, 73%, 34%, and 63%, respectively [31]. In an Italian study by Marra et al., patients with oral tongue SCC were followed after surgery. Those with minor high-risk factors for recurrence received RT alone (about 25.5%), and patients with ENE or positive surgical margin received chemoradiotherapy (17.9%). The majority of patients were T1-2 N0 (59.4%). The 5-year DFS and OS rates were 87.4% and 91.3%, respectively [32]. These findings suggest that oral tongue cancer patients can have a relatively long survival by using selected post-operative brachytherapy or external beam procedure.
Regarding RT technique, although non-significant, a trend towards better outcomes was observed by applying BT as monotherapy in early stage and as a boost in locally advanced cases compared with EBRT alone. Overall, the outcomes of BT were better than EBRT + BT boost or EBRT alone. This is in some part due to the patient selection for BT that are generally at lower risk of recurrence than those needing EBRT to cover large surgical beds or undissected or involved nodal beds. Other reasons may include the radiobiological impact of a higher dose, and a shorter time of delivery compared with EBRT. The surgical bed is a hypoxic area that is relatively resistant to radiotherapy. BT source exerts the highest dose in its vicinity around the implanted interstitial catheters. Nevertheless, based on the conventional linear-quadratic model, the calculated EQD2 dose for BT alone or BT boost was not large enough to justify better outcomes, but its lower duration of delivery, with less overall treatment time, had a great impact on tumoral cell re-population [33, 34]. By correcting the tumor re-population time in conventional EQD2 formula, the equivalent BT dose would be similar to EBRT dose of 60 Gy in 30 daily fractions. However, we should keep in mind that in interstitial BT, the dose around the catheter is infinitely high, and the lowest doses during planning are seen [21]. Although the normal tissue toxicity and tolerance were not within the scope of this study, by considering the geometrical distance and rapid dose fall-off, the superiority of BT would be even higher [35].
This study offers a valuable retrospective analysis of long-term outcomes in patients with tongue squamous cell carcinoma treated with curative radiotherapy. The strengths of the study include a clear methodology with defined patient selection criteria, comprehensive data collection of survival outcomes, and clear presentation of results. However, there are limitations inherent to the retrospective nature of the study, such as the potential for missing data or selection bias, which needs to be acknowledged. Additionally, the sample size might influence generalizability, and variations in specific treatment details within each radiotherapy group could impact results. Furthermore, the absence of data on treatment-related toxicities limits a fully comprehensive assessment of the treatment approaches.
Conclusions
Oral tongue SCC has a relatively good survival treated with surgery and adjuvant radiotherapy. Our initial findings suggest that both adjuvant EBRT and BT used as monotherapy in early stage patients or combined in locally advanced patients, are effective treatment modalities for improving the outcomes of patients with oral tongue SCC. When BT is logistically available, both in early and locally advanced stages, it may provide local control benefits. Future studies with larger, prospective cohorts are necessary to further elucidate the role of these radiotherapy techniques and include data on treatment-related toxicities, to provide a more comprehensive picture of optimizing treatment strategies in TSCC patients.