Introduction
Rheumatoid arthritis (RA), a systemic autoimmune disease, is known to cause chronic systemic manifestations and accompanying synovitis, which can lead to joint damage and disability. Given that RA has a heterogeneous phenotype and is associated with several pathogenic pathways, identification of the molecules responsible for RA pathogenesis is key for developing effective therapeutic strategies.
Monocytes/macrophages play a key role in RA progression by generating inflammatory factors within the inflamed synovium [1]. In addition, monocytes/macrophages are also central to pathological bone erosion in RA owing to the high rate at which they differentiate into osteoclasts [2]. Clec4e is a macrophage-inducible C-type lectin (MINCLE) and a transmembrane pattern recognition receptor that functions in innate immunity [3, 4]. MINCLE is predominantly expressed in monocytes/macrophages and is stimulated in several inflammatory conditions [5-10]. Nakamura et al. reported augmented MINCLE mRNA levels in bone marrow-derived mononuclear cells (BMMC) taken from patients with RA compared to osteoarthritis (OA) [10]. We previously reported MINCLE expression in synovial macrophages isolated from the synovium of OA patients, confirming that MINCLE levels may be elevated in inflammatory conditions [9]. A recent study also reported that MINCLE contributes to osteoclastogenesis [11]. Therefore, elevated MINCLE levels may contribute to the pathogenesis of inflammation and bone destruction in RA. However, MINCLE expression has not been examined in the synovial tissue (ST) of RA patients.
Here, we examined MINCLE expression in ST from RA and OA patients.
Material and methods
Patients
ST specimens were obtained from 20 RA and 20 OA patients during joint replacement surgery. All RA patients satisfied the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria [12]. Meanwhile, the OA patients were all radiographically diagnosed with unilateral Kellgren/Lawrence (K/L) grades 3 and 4 OA. This study protocol received approval from the Ethics Review Board of Kitasato University (reference number: KMEO 19-259).
Fifteen of the 20 specimens from each group were immediately subjected to analysis of MINCLE expression using quantitative polymerase chain reaction (qPCR), while the remaining five specimens were used to isolate macrophages.
Comparison of MINCLE expression in ST
Methods used for total RNA extraction from ST and isolation of macrophage cells and cDNA synthesis were based on previous studies [9, 13]. PCR primer pair sequences used for qPCR analysis were: MINCLE-sense (5'-GTG CCT GTT TCA TCA CCA GA-3') and MINCLE-antisense (5'-TTC CCA GTT CAA TGG ACA ACA A-3') for MINCLE amplification (product size: 152 bp); CD14-sense (5'-TCC CTC AAT CTG TCG TTC GC-3') and CD14-antisense (5'-ATT CCC GTC CAG TGT CAG GT-3') for CD14 amplification (product size: 150 bp); and GAPDH-sense (5'-TGT TGC CAT CAA TGA CCC CTT-3) and GAPDH- antisense (5'-CTC CAC GAC GTA CTC AGC G-3') for GAPDH amplification (product size: 202 bp). Melting curve analysis was used to examine qPCR product specificity. Relative MINCLE, CD14, GAPDH mRNA levels were determined using qPCR [9]. MINCLE and CD14 mRNA levels were normalized to that of the housekeeping gene GAPDH.
Isolation of CD14+ and CD14– cells
To examine MINCLE expression in macrophages, we extracted synovial macrophages from the ST of the knees of 5 OA and 5 RA patients. ST was digested with collagenase and the resulting cells were incubated with biotin-conjugated mouse anti-human CD14 monoclonal antibody (1 : 20; clone M5E2, BioLegend) for 30 minutes at 4°C. After washing twice with phosphate-buffered saline (PBS), the cells were added to streptavidin-conjugated magnetic particles (BD Biosciences, CA, USA) and separated in a magnetic separation system (BD IMag cell separation system, BD Biosciences) into CD14+ (macrophage) and CD14– cells, as described elsewhere [13]. Freshly extracted CD14+ and CD14– cell fractions were subjected to qPCR to analyze CD14 and MINCLE expression in OA and RA.
Statistical analysis
Normal distribution of data was confirmed using the Kolmogorov-Smirnov test, and differences in CD14 and MINCLE expression between RA and OA groups were compared using the Mann-Whitney U test. Differences in MINCLE expression between CD14– and CD14+ cells were determined using the paired t-test or paired Wilcoxon- test. Comparisons among 3 or more groups were conducted using the Kruskal-Wallis test. p < 0.05 was used to indicate statistical significance. All statistical analyses were performed using SPSS software (v. 25.0; IBM Corp., Armonk, NY, USA).
Results
MINCLE expression in ST of OA and RA patients
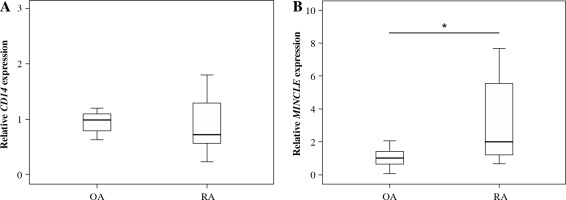
Patients in the OA and RA groups had comparable demographic characteristics, including age, male/female ratio, and body mass index (Table 1). CD14 expression was likewise similar in OA and RA patients (p = 0.561; Fig. 1A). In contrast, MINCLE expression was significantly elevated in ST from RA compared to OA patients (p = 0.005; Fig. 1B).
Table 1
Patients’ demographic characteristics
MINCLE expression in CD14+ and CD14– fractions
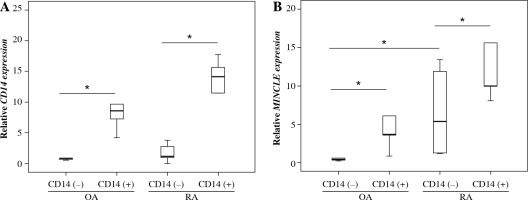
To identify the synovial cells expressing MINCLE, we examined MINCLE expression in CD14+ and CD14– cell fractions. To confirm that CD14+ cells had been successfully isolated, we also examined CD14 expression. In both the OA and RA groups, CD14 expression was significantly higher in the CD14+ cell fraction (p = 0.032 and p = 0.015, respectively; Fig. 2A). Similarly, MINCLE expression was significantly elevated in the CD14+ cell fraction in both the OA and RA groups (p = 0.043 and p = 0.043, respectively; Fig. 2B). Further, while no significant differences were observed in the CD14+ fraction between RA and OA groups, MINCLE expression was significantly elevated in CD14– fractions isolated from RA compared to OA patients (p = 0.028; Fig. 2B).
Discussion
Evidence suggests that MINCLE has crucial contributions to inflammation. For example, specific expression of MINCLE is observed in infiltrating M1 macrophages in the obstructed kidney of the unilateral ureteral obstruction mouse model of renal injury [8]. Further, Tanaka et al. demonstrated that the sensing of renal tubular cell death by MINCLE leads it to induce persistent inflammation following acute kidney injury in mice [14]. MINCLE has also been observed to have roles in neuroinflammation in cerebral ischemia and traumatic brain injury in rats [15, 16]. Here, we observed elevated synovial expression of MINCLE in RA patients. A previous study showed that injection of a MINCLE agonist induced skin inflammation in mice. Further, a MINCLE agonist induced tumor necrosis factor α (TNF-α) production in human blood monocyte-derived macrophages, which was completely suppressed by an anti-human MINCLE antibody [17]. Together, this evidence suggests that MINCLE may be a major contributor to synovial inflammation in RA.
Damage-associated molecular patterns (DAMPs) are a group of molecules that are excreted by damaged or dying cells. When released, DAMPS activate the innate immune system and have been linked to RA [18, 19]. A recent study showed that MINCLE senses osteocyte-released DAMPs and functions to enhance osteoclastogenesis [11]. Therefore, elevated levels of MINCLE in RA may play a role in bone destruction by facilitating osteoclastogenesis.
A large number of studies have reported MINCLE expression in myeloid cells [20, 21]. However, studies have also reported MINCLE expression in endothelial and neuronal cells in ischemic stroke models and stroke patients [22, 23]. In our study, we observed elevated MINCLE expression in the CD14+ macrophage cell fraction isolated from both OA and RA patients, with no significant differences in MINCLE expression between the groups. In contrast, MINCLE expression in CD14– cells was elevated in RA compared to OA patients. These findings suggest that MINCLE expression in the non-macrophage fraction may be a key feature of RA.
Our study has a number of limitations. First, we did not examine a healthy control group. Second, all samples were extracted from patients’ knees during joint replacement surgery; thus, the joints were severely damaged by the disease. Further investigation in patients with early disease is needed to reveal the importance of MINCLE in RA. Third, we did not examine the mechanism by which MINCLE contributes to RA pathology. Finally, we did not identify which cells within the CD14– fraction express MINCLE. Additional studies are needed to clarify these issues.