Purpose
Squamous cell carcinoma (SCC) of oral cavity is conventionally treated with surgery followed by adjuvant therapy, depending on post-operative histopathology. Tumors of the lip and angle of the mouth need extensive reconstruction during surgery. Two major challenges in comprehensive reconstruction of the angle of the mouth include maintaining an adequate mouth opening for food intake and preserving the competence of oral commissure to avoid drooling of food and saliva. To overcome these challenges, brachytherapy (BT) alone or in combination with external beam radiotherapy (EBRT) treatments for the lip and angle of the mouth tumors are used. Lip cancer is characterized by early detection due to its location and relatively slow progression. Only 5-10% of patients have nodal involvement at diagnosis, usually with T3-T4 stage. Occult nodal metastasis has been found in 10-12.3% of patients with T1 tumors, and up to 34.8% of patients with T2 tumors [1]. In other surgical series, the incidence of lymph nodes involvement at the time of diagnosis was even lower (7.5%) [2].
Brachytherapy via interstitial catheters has a significant advantage, i.e., very high radiation doses are delivered around the catheters, and the dose quickly falls off. This allows delivery of tumoricidal dose to primary tumor while limiting the dose to critical organs at risk (OARs), such as the skin, mandible, and sub-mandibular gland. Several studies have investigated BT alone in early-stage lip tumors, most of which were limited to T1N0. However, given the risk of 7.5-35% of nodal involvement in T2N0M0 tumors, BT alone may not provide adequate long-term loco-regional control [1, 2]. Few studies have investigated whether a BT boost in conjunction with EBRT at a prophylactic dose of 44-50 Gray (Gy) provides adequate loco-regional control. The American Brachytherapy Society (ABS) Task Group report advocates the combination of EBRT and BT for tumor sizes of 30-40 mm, and the use of BT as the sole modality for tumors smaller than 30 mm [3].
With this in mind, our institutional protocol follows a combined approach of BT and EBRT in selected T2N0M0 cases. However, dose summation remains a major challenge. Conventional dose summation based on linear quadratic (LQ) model, where the doses of BT and EBRT are converted to 2 Gy equivalent doses (EQD2) and biological equivalent doses (BED), has been used for a long time [4, 5]. This approach takes into account factors, such as dose-rate, dose per fraction, and tissue radiobiology, to obtain a composite dose from all treatment modalities. The continued use of different techniques in EBRT, especially VMAT, where significantly more geometric, spatial, and intra-tumoral dose heterogeneity comes into play, makes dose calculation more challenging. The limitation of such a dose calculation is that it does not provide geometric and spatial distribution as well as a summation of doses in the tumor and adjacent OARs. Therefore, the summation of maximum doses (Dmax) received by OARs may not correspond exactly to the actual Dmax, as the location of Dmax may vary in EBRT and BT. This limits the potential for dose escalation. Using different software in BT and EBRT treatment planning system (TPS) further complicates the dose summation process. Therefore, there is a need for a unified system that can incorporate the plans and provide a combined plan summation with accurate spatial distribution, which can potentially enable better delivery of radiotherapy (RT) doses.
Osteoradionecrosis (ORN) and superficial skin necrosis are the major morbidities associated with BT. Skin ulcers are rare at doses < 50 Gy, but increase to 5-8% at doses between 60 and 100 Gy EQD2 [6]. Even though ORN is infrequent in EBRT alone, it becomes a serious morbidity when treated with BT alone or in combination (BT + EBRT). Peterson et al. reported a weighted ORN prevalence of 5.3% when using BT [7]. Therefore, avoiding a high-dose sleeve, especially near critical OARs, such as the mandible, skin, and sub-mandibular gland as well as optimizing EBRT doses in the area of high-dose sleeve, are of utmost importance to reduce RT-induced morbidity.
Utilization of deformable image registration (DIR) system solutions, such as Velocity® software, can solve this problem. DIR systems use multiple image sets, where one set serves as the base, and the other image sets are deformed to match the base image, using mutual information with an elastic B-spline optimizer. Furthermore, the plans generated in different image sets are used, and dose distribution is deformed identically to DIR to obtain a composite dose distribution. DIR-based dose summation has already been used in various sites, e.g., cervix, but there is a lack of data on its application in the head and neck region. Using this software, we retrospectively evaluated our patients treated with a combined BT and EBRT approach.
This study aimed to establish and evaluate the dosimetric parameters of PTV and OARs with combined doses of high-dose-rate BT (HDR-BT) and EBRT in early T1-T2N0 tumors of the labial and buccal mucosa, using DIR software (Velocity®). The combined EQD2 calculated manually using LQ model-based calculation (EQD2-D90 BT + EQD2-D98 EBRT) was compared with the doses estimated using a DIR-based plan sum calculation. In addition, this study reported the combined maximum dose to the mandible, skin, and sub-mandibular gland, calculated by the manual EQD2 dose summation and DIR-based dose summation.
Material and methods
The present study was a retrospective analysis conducted at a tertiary care center, based on dosimetric data of patients treated between January 2021 and November 2023. This analysis was performed in patients with histopathologically proven T1-2N0M0 SCC of the lip and angle of the mouth, who were between 18 and 80 years old, both sexes, had a Karnofsky performance status of 80 or higher, were eligible for general anesthesia and BT, and underwent an interstitial BT boost followed by EBRT.
According to the institutional protocol, all selected patients underwent contrast-enhanced computed tomography (CECT) of the face and neck or high-resolution ultrasound of the neck to exclude nodal involvement, and non-contrast computed tomography (CT) of the thorax to exclude lung metastases after histopathologic confirmation. Based on clinical and radiological findings, BT feasibility in terms of its location, accessibility, and pre-anesthetic clearance, was assessed in all patients in TNM stage T1-2N0M0 of the lip and angle of the mouth. Dental prophylaxis was mandatory before radiotherapy. Depending on the extent of the disease, these patients underwent interstitial BT by insertion of a unilateral or bilateral catheters under general anesthesia.
BT contouring and treatment planning
For contouring and treatment planning, CT images were acquired on a CT simulator (SOMATOM Confidence with 80 cm bore, Siemens Healthcare GmbH), with a slice thickness of 1 mm and a resolution of 512 × 512 voxels per slice. Images were imported into Oncentra Brachy planning system (v. 4.6, Elekta BT, Veenendaal, The Netherlands), and BT treatment was performed using Flexitron afterloader unit with a cobalt-60 (60Co) source. Target volume and OARs were delineated and subsequently, catheters were reconstructed and the dose was prescribed at the basal point generated by TPS, depending on the geometry of the implant. The center of a triangle was used as the point of prescription, where catheter arrangement was in a triangular shape. Since many of the cases used 2 plane implants, in which the catheters were placed equidistant (kept 1 cm apart in each plane and at 1 cm distance between the 2 planes, to create a square-shaped or box-like distribution). In these cases, the point of prescription was taken as the midpoint between the 2 catheters, in different planes.
Gross tumor volume (GTV) was contoured on each slice using clinical and radiological assessment. Clinical target volumes (CTVs) were created by an isotropic expansion of 10 mm to fulfill the topological and anatomical requirements. It was cropped from the bone and skin. Since only T1 and T2 tumors without obvious skin involvement were included, the skin was considered as OAR and CTV was cropped out of the skin. Planning target volume (PTV) was kept the same as CTV. All BT plans were normalized, so that the minimum dose received by maximally irradiated 90% volume (i.e., D90) was 100% of the prescribed fraction dose. Furthermore, after the Paris system-based dose prescription, manual dwell-point and local geometric optimization were done to improve the dose distribution, reduce hot spots, and limit high-dose sleeves. However, no inverse dose optimization was performed. BT was administered through interstitial needles at a dose of 3.5-4.0 Gy per fraction, twice daily, with an interval of at least 6 hours between two fractions. After completion of BT, catheters were removed, and patients were discharged.
EBRT contouring and treatment planning
Two weeks post-BT, contrast-enhanced CT simulations with axial CT slices of 1 mm thickness of the selected cases were obtained. The image data sets were transferred to the Varian Eclipse system. Target volumes and OARs were contoured. GTV and CTV contours were the same as for BT, but the extent of the disease before BT was considered while contouring CTV for EBRT, as the tumor had regressed significantly in most of the cases. A low-risk CTV was created to include nodal sites at risk when the risk of nodal involvement was significant. The CTV was isotropically extended by 5 mm to create PTV and compensate for geometric uncertainties. The PTV was truncated 3 mm below the contoured body surface to account for buildup. The mandible, skin, sub-mandibular gland, spinal cord, brainstem, and bilateral parotid glands were contoured for each patient. EBRT was planned using the Rapid Arc® technique, and a dose of 44 Gy was delivered to the PTV in 22 fractions, using 2 Gy per fraction and five fractions per week.
DIR and dose summation
Computed tomography images and the respective treatment plans for both BT and EBRT of all patients were imported into the Velocity® software (version 4.0, Varian Medical Systems, Palo Alto, CA, USA) from Oncentra and Eclipse planning systems, respectively. The Velocity software is capable of performing various functions, including dose deformation, dose scaling, and dose summation with/without DIR. In this study, dose scaling and dose summation with DIR were used. Both BT and EBRT dose distributions were scaled by Velocity to an equivalent dose distribution of 2 Gy per fraction. EBRT physical dose was already 2 Gy per fraction, but since the Velocity software was unable to sum a scaled dose and physical dose, both BT and EBRT dose distributions were re-scaled. Using DIR, dose distribution determined during BT planning was summed with EBRT dose distribution, resulting in a new summed dose distribution in the form of EQD2. The summed image set was evaluated for the dose received by critical OARs, such as the mandible and skin. Since all selected patients had tumors of the labial or oral mucosa, the mandibular gland was evaluated, but was not considered a critical OAR due to its distance from the catheters.
The mandible and mandibular gland were contoured and evaluated with two indices to assess the efficiency of image registration by the DIR software, using Dice similarity coefficient (DSC) and mean distance to agreement (MDA). Since MDA is a poor index for examining under or over-contouring, DSC was also utilized to evaluate the contours. MDA tends to decrease with increasing agreement between contour sets, while the opposite is true for DSC.
Statistical methods
Statistical analysis was performed using SPSS software (version 24.0, IBM). Mean and standard deviation were estimates for quantitative data. Mean cumulative dose received by OARs were compared with EQD2-based manual dose summation and DIR-based dose summation using paired t tests. Statistical significance was determined using p values, with p < 0.05 considered significant for all analyses.
Results
Patient characteristics
Ten histopathologically proven cT1-2N0M0 cases of SCC of the lip and angle of the mouth lesions were selected. The patient details are shown in Table 1. Except for three cases, all patients were younger than 65 years old at presentation. Age ranged from 30 to 69 years, with a mean age of 56.4 years (SD ± 14.47) and median age of 57 years. Seven of the patients were males, and three were females. In seven cases, the tumor was located on the lips, and in three cases, on the buccal mucosa (angle of the mouth). All patients had T1-2N0M0 disease (T1: n = 6, and T2: n = 4). The tumor size was 3-4 mm in 6 patients, and 20-30 mm in 4 patients.
Treatment characteristics
Initially, all patients received an interstitial HDR-BT boost, and then EBRT. The BT dose in 9 patients was 21 Gy in 6 fractions, 3.5 Gy per fraction, while one patient received 20 Gy in 5 fractions, 4 Gy per fraction. All patients were treated twice daily, with an interval of 6 hours between each fraction. The EBRT dose ranged from 40 Gy to 46 Gy, 2 Gy per fraction, with the median dose of 44 Gy, 2 Gy per fraction. The patient who received an EBRT dose of 40 Gy, received 20 Gy in 5 fractions of BT. Volumetric modulated arc therapy (VMAT) was used for EBRT in 8 patients (80%), while three-dimensional conformal radiotherapy (3D-CRT) was applied in 2 patients (20%). Neck nodes were irradiated in all patients, with bilateral neck treated in six patients (60%). The mean PTV-BT volume was 10.56 cc (SD ± 4.56), and the median volume was 9.7 cc, with a range of 2.2-17.7 cc. The details of the treatment are shown in Table 2.
Table 2
Treatment details and dosimetric parameters
Dosimetric results
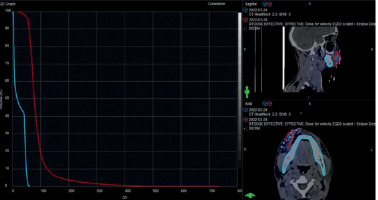
The median equivalent dose in 2 Gy fraction (EQD2) of D90 of PTV-BT was 19.7 Gy (range, 7-22.9 Gy) (Tables 2, 3, Figure 1). The DIR-based spatial dose summation between BT and EBRT provided the median summated PTV-BT D90 (DIR) as 56.3 Gy (range, 39.8-70.0 Gy), and PTV-BT D98 (DIR) as 39.6 Gy (range, 19.0-66.2 Gy) (Table 4). Our choice of D90 for BT was based on the Groupe Européen de Curiethérapie European Society for Radiotherapy and Oncology (GEC-ESTRO) guidelines, which recommend that D90 is more reliable than D98 for dose reporting in BT due to the rapid dose fall-off [8]. Also, a change in application geometry has a greater impact on D98 than on D90, making D90 a more reliable parameter for dose calculation. On the other hand, D98 is considered a reliable indicator of dose constraint due to the relatively uniform dose distribution in EBRT, where there is no rapid dose decrease. This was endorsed in the report of the International Commission on Radiation Units and Measurements Report 83 [9].
Table 3
Case-wise prescribed and calculated dose details
Table 4
Various dosimetric parameters of planning target volume (PTV) treated with external beam radiotherapy (EBRT) + brachytherapy (BT)
As for the mandible, the median EQD2 of the maximum dose (Dmax) received by the mandible from BT and EBRT was 3.15 Gy (range, 1.2-3.7 Gy) and 47.2 Gy (range, 46.7-55.5 Gy), respectively. Therefore, the manual EQD2-based dose summation yielded the median summed mandibular Dmax of 50.35 Gy (range, 45.8-52.3 Gy), while DIR yielded the median summed dose of 64.6 Gy (range, 49.6-89.0 Gy), with the minimum dose received by maximally irradiated 2 cc volume (D2cc) being 50.6 Gy (range, 43.5-54.1 Gy). There was a statistically significant difference in Dmax between data obtained from the dose summation and DIR-based dose summation (64.79 Gy vs. 49.98 Gy, p = 0.001). The median percentage of the mandible that received a dose of at least 60 Gy according to DIR was 0.09% (range, 0.0-16.2%) (Table 5).
Table 5
Various dosimetric parameters of mandible treated with external beam radiotherapy (EBRT) + brachytherapy (BT)
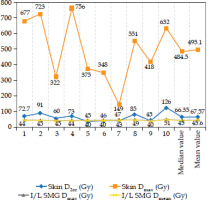
The median DIR-based spatial dose summation of the skin D2cc and D0.2cc (minimum dose received by maximally irradiated 0.2 cc of the skin) between BT and EBRT was 66.4 Gy (range, 40-126 Gy) and 151 Gy (range, 55-404 Gy), respectively. Moreover, for the ipsilateral sub-mandibular gland, the median DIR-based dose summation of Dmean and Dmax was 45 Gy (range, 40-51 Gy) and 47 Gy (range, 41-53 Gy), respectively (Figure 2). The median Dmax for the spinal cord was 42.3 Gy, and for the brainstem was 28 Gy. The median Dmean for the left parotid gland was 22.6 Gy, and 18.2 Gy for the right parotid gland. Most of the doses received by the spinal cord, brainstem, and bilateral parotids, were contributed by EBRT, with insignificant contribution from brachytherapy.
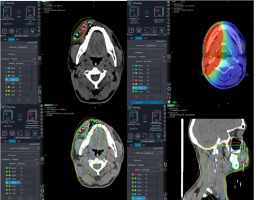
Dice similarity coefficient for the mandible ranged from 0.68 to 0.89, with the mean and median DSCs of 0.83 and 0.86, respectively. MDA for the mandible ranged from 0.8 mm to 1.48 mm, with the mean and median values of 1.08 mm and 0.97 mm, respectively. The median DSC for the left sub-mandibular gland (SMG) was 0.72 (range, 0.39-0.81), and the median MDA was 1.79 (range, 1.32-2.7). The median DSC and MDA for the right SMG were 0.74 (range, 0.49-0.84) and 1.72 (range, 1.28-2.78), respectively. Therefore, the registration agreement between the EBRT and BT planning CT images for the mandible was the highest of the three OARs selected for this comparison. The dose distributions for EBRT, BT, and combined DIR base summation are shown in Figure 3.
Discussion
For early tumor of the lip and angle of the mouth, surgery involves extensive reconstruction, leading to difficulties in maintaining adequate mouth opening and integrity of the oral mucosa to prevent food and saliva leakage. Several studies have demonstrated clinical benefits of interstitial BT alone or with EBRT as a boost for T1-T2N0 cancers of the lip and angle of the mouth, in organ and functional preservation and better cosmetic outcomes.
In the 2017 update of the GEC-ESTRO’s Advisory Committee on the Practice of Radiation Oncology (ACROP) recommendations, BT alone is endorsed as an acceptable treatment modality for intact T1 and early T2 lesions with low-risk of lymph node involvement, meeting at least one of the following conditions: patient preference, tumor in a functionally important region, a cosmetically important region, conditions for medical inoperability, or small recurrent tumors in irradiated areas not suitable for surgical salvage. The group suggested doses of 36 Gy, 45 Gy, and 48 Gy, administered twice daily at 4 Gy, 5 Gy, and 6 Gy per fraction, respectively, 5 days per week [10, 11]. Also, the recommendation is provided for selective treatments of the neck, if the lip tumor is larger than 2 cm or if the commissure or skin is affected.
Combined EBRT and BT treatment was considered acceptable for intact T1-T2 tumors in medically inoperable patients with a high-risk of lymph node involvement. T3-T4 and/or N+ tumors requiring surgery, and associated with unacceptable functional or cosmetic defects were also considered candidates for combined EBRT and BT treatment. Usual dose recommendations are 40-45 Gy EBRT, followed by a BT boost of 18 Gy or 21 Gy, given twice daily, at 3 Gy or 3.5 Gy per fraction [12-14]. The 2017 ABS guidelines recommended BT alone for the lip and angle of the mouth tumors of less than 3-4 cm in size, without lymph node metastases and no bony involvement. EBRT with BT boost was suggested for tumors, which cannot be operated and are 3-4 cm in size, have < 1 cm invasion into the gingiva, and no bony infiltration. The guidelines mention invasion of more than 1 cm into the gingiva and bony infiltration as contraindications for BT [3]. According to the GEC-ESTRO suggestions for SCC in the head and neck region, BT is not recommended if tumor extends to the intermaxillary commissure or bucco-alveolar sulcus [15].
The 2014 Indian Council of Medical Research (ICMR) guidelines for the treatment of oral cavity cancers recommend BT with an HDR system (dose, 48 Gy in 12 fx., 4 Gy BD × 6 days) as a safe treatment for highly compatible patients with accessible and superficial well-differentiated early lesions, preferably T1, with negative ultrasound-confirmed nodal findings, and located at some distance from the bone. Early lesions, which are not candidates for BT alone can be treated with 45-50 Gy EBRT, followed by a 20-25 Gy low-dose-rate (LDR) BT boost or an equivalent HDR system [16]. The 2020 guidelines of the Indian Brachytherapy Society (IBS) recommend a BT boost (16-26 Gy in 4-6 fx.) combined with EBRT (40-46 Gy in 20-23 fx.) for infiltrative lip and angle of the mouth lesions larger than 2 cm, which may involve lymph nodes. Anteriorly located early lesions of less than 2 cm with involvement of the angle of the mouth, with more than 5 mm mandibular margin and free superior and inferior gingivobuccal sulcus, may also be considered good candidates for radical BT alone [17].
The most commonly used techniques for BT in cancers of the lips and buccal mucosa are rigid needles or plastic tubes. As far as the lip is concerned, the rigid needle approach using a template offers optimal geometric conditions for the implantation procedure, which is why it is strongly recommended for HDR treatments. For tumors affecting the upper lip or involving the commissure, the plastic tube technique may offer better treatment results [15]. Our team has used a flexible implant with the plastic tube application technique. Based on the above guidelines, our institute follows the protocol of using a BT boost followed by EBRT for T2 tumors of the lip and angle of the mouth. This sequence helps us to avoid needle insertion against the background of oral mucositis after EBRT, ensuring that the implanted lesion is well assessable, as the lesion might regress or disappear after EBRT.
The most difficult part of using a combination of EBRT and BT is the dose summation of EBRT and BT. Due to the spatial differences between the two treatment scans and the use of different software for planning, simply adding the EQD2 of Dmax to a given OAR may not be representative of the actual Dmax. This can be addressed by using rigid or deformable image registration. Image registration is about finding the best spatial transformation between the same points in two image sets. The transformation in rigid registration maintains the separation between each point in the image by providing only translation and rotation. The deformation of soft tissue during BT due to catheter implantation limits the optimal matching of image sets in rigid image registration (RIR) [18]. RIR, with the bony anatomy as a reference, can also lead to sub-optimal matching due to changes in tumor position and tumor volume due to applicator placement as well as rapid response in the tumor because of a very high-dose in the middle of catheters [19]. The lack of immobilization devices in BT scanning can lead to large positional uncertainties compared with EBRT scanning [20, 21]. In the current study, a comparison of DIR-based dose summation and EQD2 revealed significant differences. This indicated that adding up the Dmax or Dmean after converting BT doses to EQD2 is not an ideal method per se, and there is a need for actual spatial dose summation.
The transformation in DIR, on the other hand, is spatially variable and retains the pixel-pixel relationships. DIR establishes a spatial correspondence between different image sets, and accounts for anatomical differences between EBRT and BT-CT scans. In this way, the doses in each tissue voxel from each of the treatment modalities can be tracked [22]. It is used for various purposes, including functional imaging, adaptive offline planning in EBRT (automatic segmentation and contour propagation to avoid re-simulation and re-planning), and dose accumulation (calculation of the actual dose delivered to the patient in each treatment fraction, or when combining two different treatment modalities). The DIR software used in our study was Velocity®, which can be used for dose shaping, dose tracking, dose scaling, and dose accumulation using the B-spline algorithm. Dose scaling and dose summation with DIR functions of the Velocity software were employed in this study. Accurate dose summation would provide an idea of the total accumulated dose, and help in planning dose escalation and optimization to avoid overdose of OARs. To the best of our knowledge, no attempts have been made to sum the dose from EBRT and BT in head and neck sub-regions using this algorithm; the current study is the first research on this subject.
So far, the use of DIR in practice has been mostly limited to cervical cancer. The ABS and GEC-ESTRO currently recommend dose summation for EBRT and HDR-BT using EQD2 summation based on the LQ model, and have created a separate worksheet for this purpose [23]. Linear summation of doses is based on two assumptions, i.e., uniform dose distributions for pelvic EBRT delivered at a single dose level, and each BT implantation providing reproducible geometric, spatial, and dosimetric parameters for OARs [8]. However, this does not work if the EBRT plan includes a boost (pelvic or para-aortic nodal boost), or if EBRT is performed using highly conformal techniques with a simultaneous integrated boost (SIB) to treat multiple targets. Rigid image registration was found to be of limited use in this context, as the change in OARs between BT fractions resulted in inaccurate summation [24]. Several studies have been conducted to compare EQD2 summation with DIR-based dose summation in cervical cancer. Kim et al. found that Velocity software-based DIR dose summation resulted in higher values than EQD2 addition, especially when SIB was used during EBRT [25].
Teo et al. evaluated cumulative D2cc doses to the bladder and rectum for both EBRT and intra-cavitary BT using the MIM Maestro software, and observed no significant difference between the results obtained by DIR and parameter addition (EQD2 addition) [26]. However, the summation of fractionated BT doses differed significantly between the two methods. Clinical utility of intensity-based DIR algorithms remains uncertain for contours with poorly defined segmentation boundaries and in regions, where PTV contour changes significantly and abruptly from one image set to another.
Another concept used in EBRT and BT dose summation is the equivalent uniform dose (EUD) method. This approach calculates a single dose equivalent that represents the combined effect of different doses and dose distributions for diverse treatment modalities. By accounting for differences in dose distribution and biological responses, the EUD method provides a consistent metric for dose summation in radiotherapy. It is applicable to both targets and OARs, so it can be used to compare and optimize treatment plans with different RT modalities. A similar concept is used in the uniform dose convolution (UDC) method, which (unlike the EUD method) assumes a uniform EBRT dose distribution, so that the entire target and nearest OARs receive the whole prescribed dose. It applies primarily to 3D-CRT plans, and calculates a single uniform dose that represents the combined effect of different doses and dose distributions across diverse treatment modalities [27, 28]. Other approaches, such as rigid registration, have also been investigated in several studies.
The use of DIR in cervical cancer is important due to location of the tumor, differential filling of adjacent OARs, and the use of BT with multiple fractions. Our approach to apply a similar concept in the head and neck region was primarily aimed at minimizing radiation-induced morbidity, such as ORN. ORN is a rare complication after RT that leads to necrosis of bone tissue and impaired healing. Its incidence rate is 9% in patients who received a radiation dose of more than 60 Gy [29]. V60 (volume of more than 60 Gy received) > 14% is a significant predictor of ORN [30]. Regarding sub-mandibular gland (SMG) toxicity, xerostomia after radiotherapy can be attributed to the parotid glands, SMG, and minor salivary glands. According to a systemic analysis by Jensen et al., the prevalence rates after 2 years of RT with conventional RT, 3D-CRT, and IMRT, were 90.9%, 69.4%, and 68.1%, respectively [31]. Sparing the SMG significantly improves the preservation of unstimulated salivary flow compared with cases, where only the parotid gland is spared, leading to a significant reduction in xerostomia [32]. A mean dose of ≤ 39 Gy has been estimated for potential restoration of SMG function over time [33]. Surgical relocation of the SMG to a sub-mental space (outside the radiation field) helps preserving its function and prevents radiation-induced xerostomia [34].
Up to 95% of patients with head and neck tumors experience varying degrees of skin toxicity [35]. Hyperpigmentation develops within two to four weeks after conventionally fractionated radiotherapy, corresponding to a cumulative radiation dose of 20 Gy. Hyperemia and edema occur at radiation doses of 30 to 40 Gy, which can lead to epilation and moist desquamation at higher doses of 45 to 60 Gy [36]. In a retrospective study among 9 patients with early carcinoma of the lip and buccal mucosa treated with surface mold BT, Mukherji et al. showed a grade 1 skin reaction in 89% of cases, and a grade 2 response in 33% of cases [37]. Similar results were reported by Unetsubo et al., who examined 17 patients with early oral cavity carcinomas, mainly of the lip and buccal mucosa, treated with EBRT followed by surface mold BT boost, and found grade 2 skin toxicity in 35% of cases [38].
In the present study, MDA and DSC were evaluated as matrices to assess the accuracy of image registration. MDA values were estimated for the mandible and SMG structures. Since the mandible is a bony structure, the difference in contrast between the surrounding tissue and the mandible is sufficient to allow relatively accurate delineation. However, in the case of SMG, the gray values have a lower contrast with the background, resulting in greater uncertainty in MDA values evaluated for SMG. In addition, MDA may not be able to distinguish between under and over-contouring; therefore, another metric, DSC, was evaluated [39].
Our analysis of 10 patients showed that the Dmax of the mandible calculated with DIR was between 49.6 and 89 Gy, but the EQD2-based dose summation was only between 45.8 and 52.3 Gy. In seven out of 10 patients, Dmax of the mandible was above 60 Gy. However, only one patient had a V60 Gy mandible of more than 14%. None of the patients developed ORN by their last follow-up. The main advantage of dose summation, especially for lip and oral cavity tumors, is the possible dose escalation or dose limitation to avoid damage to OARs. Prospective use of DIR-based dose summation allows optimization of the dose to the mandible, which in turn can reduce the risk of ORN as well as optimization of the skin dose that can reduce grade 3 or 4 skin reactions. Prospective use of DIR can optimize the dose component of BT at the time of EBRT contouring and dose constraint setting. With the IMRT/VMAT technique, where the mandible and skin are contoured as OARs and the dose is optimized for the received BT dose, good sparing of the mandible and skin can be achieved. However, if BT is performed after EBRT, DIR-based dose summation can be used as a guide for preferential irradiation and manual optimization to keep a 150-200% isodose line away from the mandible and skin. This can be done prospectively and can potentially reduce morbidity, such as ORN and grade 3-4 skin toxicity.
The current study has an inherent limitation of being a retrospective study with a small sample size (n = 10), which limits its generalizability. In our further studies, we intend to apply the method prospectively with a larger cohort. In addition, the DIR method of dose summation is highly dependent on the quality of the contours, and may be inaccurate in regions with significant anatomical changes. An independent review of the contours by several radiation oncologists to avoid strong contour changes and a cross-verification of the images obtained with the DIR method, could minimize this disadvantage.
Future directions
A combination of biologically adapted dose mapping and voxel-based deformation for accurate dose accumulation may be the way forward. However, such trials require an adequate clinical validation. Based on our study results, the prospective use of DIR with optimization in either EBRT with IMRT or BT with manual or graphic optimization, may yield better results.
Conclusions
Deformable image registration software-based dose summation in combined EBRT and BT in the head and neck region is feasible. It allows accurate summation of the dose delivered to the target and OARs by the two modalities and, if performed prospectively, can potentially allow the optimization of threshold doses for critical OARs, reducing post-treatment morbidity. Our initial trial demonstrated promising results, but prospective validation with a larger sample size is required for broader generalizability.