Introduction
Knee osteoarthritis (KOA), a degenerative joint disease, is commonly typified by articular cartilage degeneration, osteophytosis, and bone remodeling in addition to fibrosis and synovial hyperplasia [1]. Additionally, increasing evidence indicates that synovial inflammation is involved in a number of the signs and symptoms underlying KOA, such as joint effusion and swelling. Synovial inflammation in KOA is likely to contribute to disease progression along with the progression of structural changes in KOA [2]. While synovium inflammation is a key player in KOA pathology, the molecular signals responsible for the regulation of synovial inflammation have not been fully determined.
The macrophage-inducible C-type lectin (Mincle) Clec4e is a transmembrane pattern recognition receptor with roles in innate immunity [3, 4]. Mincle is predominantly expressed in monocytes/macrophages and is stimulated in several inflammatory conditions [5-11]. Human blood monocytes and bone marrow mononuclear cells (BMMCs) obtained from rheumatoid arthritis (RA) patients showed higher expression of MINCLE than the healthy control sample [10, 11]. Previous studies have shown that there are high levels of Mincle in macrophages in adipose tissue from obese humans and mice [6] and in a cisplatin-induced renal injury murine model [7].
The synovial tissue (ST) is a highly specialized structure with multiple functions. It comprises a thin but highly cellular lining layer of synovial fibroblasts and synovial macrophages [12], in addition to a second layer. Macrophages are one of the major sources of inflammatory signals in the ST of KOA patients and may therefore contribute to joint destruction and pain [13-15]. However, the expression of Mincle has not been examined in synovial macrophages from KOA patients.
A number of recent studies suggest that tumor necrosis factor alpha (TNF-α) levels are increased in ST, and that this inflammatory signal is associated with KOA pathology [13, 14, 16, 17]. Synovial macrophages, in particular M1 macrophages (classically activated macrophages), produce TNF-α and induce the expression of matrix metalloprotases and a disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS4) in synovial fibroblasts extracted from a KOA mouse model and KOA patients [14, 15]. Moreover, several inflammatory stimuli have been shown to induce high levels of Mincle in macrophages [5, 8, 9] to contribute to maintaining the inflammatory state [8]. Based on these findings, we speculated that TNF-α may regulate Mincle expression in synovial macrophages.
Here, we investigated Mincle expression and regulation in the ST of KOA patients.
Material and methods
Patients
This study included 19 subjects with radiographic KOA (unilateral Kellgren/Lawrence [K/L] grades 3-4) who received total knee arthroplasty at our institution. Among them, there were 4 men and 15 women aged 56–85 years (mean ±standard deviation [SD], 72.2 ±7.0 years) with a mean ±SD body mass index (BMI) of 25.5 ±3.2 kg/m2 (range 18.8-30.9). An ST sample was extracted from each operated knee during surgeries. This study was approved by the Ethics Review Board of Kitasato University (refe-rence number: KMEO B13-113). All participants gave consent one day prior to the surgery for the extraction and use of their ST.
Immunohistochemistry
To examine the distribution of Mincle, ST samples were fixed in 4% paraformaldehyde (PFA) formalin in phosphate buffered saline (PBS) (Wako Pure Chemicals, Osaka, Japan) for 48 h. PFA-fixed ST samples were embedded in paraffin and sectioned at 4 µm thickness before being deparaffinized in a xylene replacement solvent (Clear-plus®, Pharma, Tokyo, Japan) for 1 hour. The sections were hydrated in a series of decreasing concentrations of ethanol (100%, 95%, 80%, and 75%) and subsequently rinsed in distilled water. The sections were incubated with a mouse anti-Mincle monoclonal primary antibody (1 : 200; clone AT16E3, GeneTex, LA, USA) and mouse anti-TNF-α monoclonal primary antibody (1 : 100; clone 2C8, Abcam, Cambridge, UK) for 6 hours at 4°C.
Flow cytometric analysis
Flow cytometric analysis was performed to identify the ST cells expressing Mincle [13]. ST were digested with α-minimum essential medium (α-MEM, Invitrogen, CA, USA) containing 1 mg/ml collagenase solution (Sigma, MO, USA) on shaker for 2 hours at 37°C. Extracted cells were incubated with phycoerythrin (PE)-conjugated mouse anti-CD14 monoclonal antibody (1 : 20; clone M5E2, Biolegend, CA, USA), fluorescein isothiocyanate (FITC)-conjugated mouse anti-CD45 monoclonal antibody (1 : 20; clone LCA, BD Biosciences, San Jose, CA, USA), allophycocyanin (APC)/Cy7-conjugated anti-human CD31 (1 : 20; clone WM59, Biolegend), APC-conjugated mouse anti-CD90 monoclonal antibody (1 : 20; clone 5E10, Biolegend), and rat anti-Mincle monoclonal antibody (1 : 200; clone 1H2, MBL, Nagoya, Japan) for 45 minutes at 4°C. The cells were washed twice in PBS, resuspended in ice cold PBS and reacted with brilliant violet 421-conjugated goat anti-rat IgG polyclonal antibody (1 : 100; clone Polu4054, Biolegend). After washing twice in PBS, the cells were analyzed by flow cytometric device (FACSVerseTM; BD Biosciences). Isotype controls were used as the posi-tive gate.
Isolation of CD14-positive (CD14+) and CD14-negative (CD14−) cells
To examine the role of TNF-α in regulating Mincle expression, synovial fibroblasts and macrophages were extracted from the ST from the knees of 10 KOA patients. Following collagenase digestion of ST, the extracted cells were incubated with biotin-conjugated mouse anti-human CD14 monoclonal antibody (1 : 20; clone M5E2, Biolegend) for 30 minutes at 4°C. The cells were subsequently washed twice with PBS, combined with streptavidin-conjugated magnetic particles (BD Biosciences, CA, USA), and separated in a magnetic separation system (BD IMagTM cell separation system, BD Biosciences) into CD14+ and CD14- cells, as described elsewhere [13]. CD14+ and CD14- cell fractions from five patients were immediately analyzed for TNF-α, CD14, and Mincle expression using quantitative polymerase chain reaction (qPCR), while those from the remaining five patients were cultured for 7 days in six-well plates containing α-MEM. The cells were subsequently stimulated with vehicle (α-MEM), 10 ng/ml human recombinant TNF-α (Biolegend), or 10 ng/ml TNF-α + 1 µg/ml anti-TNF-α antibody (clone Mab11, Biolegend) for 24 hours, before isolating RNA for real time (RT)-PCR analysis for Mincle expression.
Statistical analysis
Differences between CD14− and CD14+ cells were examined using the t-test. Comparisons between multiple groups were performed using one-way analysis of variance with Tukey’s multiple comparisons test. For all analyses, p < 0.05 indicated statistical significance. All statistical analyses were conducted using SPSS software (v. 25.0; SPSS, Chicago, IL, USA).
Results
Localization and expression of Mincle in ST of KOA patients
Immunohistochemical and flow cytometric analyses of Mincle and TNF-α were performed to identify the cell type(s) within the synovial lining layer of the knees of KOA patients expressing Mincle (Figures 1 and 2). Both TNF-α-positive and Mincle-positive cells were observed in the intimal lining layer (Figure 1A–D). Flow cytometric analysis showed that some CD45+CD14+ macrophages were Mincle positive, while CD45–CD14– fibroblasts were Mincle negative. Quantitative PCR analysis demonstrated that the expression of MINCLE and TNFA was significantly greater in CD14+ fractions than in CD14– fractions (Figure 3, p = 0.010).
Fig. 1
Immunolocalization of Mincle and TNF-α in synovial tissue. Immunolocalization of Mincle (A, B) and TNF-α (C, D). Scale bars = 100 μm
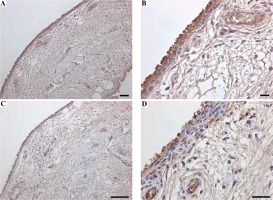
Fig. 2
Flow cytometric analysis of MincLE expression in CD14-negative and -positive synovial cells extracted from knee osteoarthritis patients. A) Dot-plot analysis of CD45+CD14+ cells and CD45–CD14– cells. X-axis, CD45; y-axis, CD14. B, C) Histogram analysis of MincLE expression in the CD45+CD14+ (B) and CD45–CD14– (C) gated regions in (A)
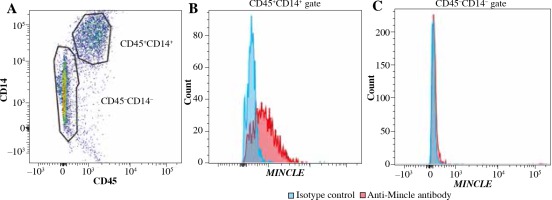
Fig. 3
Quantitative polymerase chain reaction (qPCR) analysis of CD14, MincLE, and TNF-α mRNA levels in CD14-negative and -positive synovial cells extracted from knee osteoarthritis patients. A) CD14, B) MincLE, C) tnFa. Data indicate mean ±standard error (SE) (n = 8). *Statistical difference between CD14-negative- and -positive cells
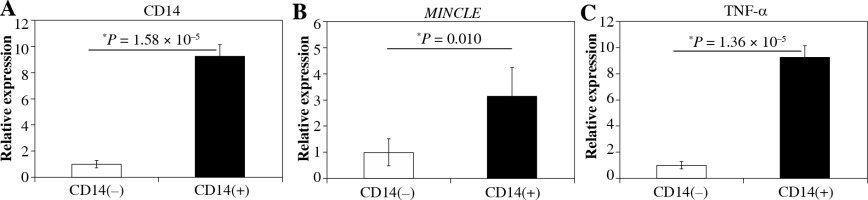
Fig. 4
Impact of tumor necrosis factor (TNF)-α on MincLE expression. CD14-positive synovial cells were stimulated with vehicle (α-minimum essential culture medium (α-MEM)], 10 ng/ml TNF-α or 10 ng/ml TNF-α + 1 μg/ml TNF-α neutralizing antibody (TNF-α + Neu) for 24 h before the extraction and analysis of total RNA. Differences between control and TNF-α and between TNF-α and TNF-α + Neu groups were assessed using the Tukey’s multiple comparisons test with one-way analysis of variance
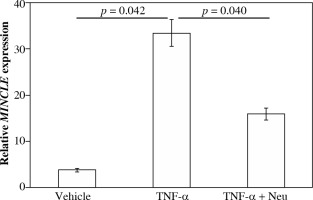
Effect of TNF-α on MINCLE mRNA expression in synovial macrophages
Stimulation of cultured CD14+ fractions with exogenous TNF-α significantly raised MINCLE mRNA expression compared to vehicle-stimulated controls (8.9-fold, p = 0.042). In contrast, stimulation with TNF-α neutraliz-ing antibody significantly reduced MINCLE mRNA expression compared to exogenous TNF-α-stimulated cells (p = 0.040).
Discussion
We demonstrated that Mincle-positive cells were localized to the synovial lining layer of the knees of KOA patients. CD45+CD14+ cells were Mincle positive, and CD14+ synovial cells and isolated CD14+ cells expressed high levels of MINCLE compared to CD14– cells. Moreover, stimulation of CD14+ cells with TFN-α induced Mincle expression. Taken together, these results suggest that Mincle may play an important role in synovial inflammation mediated by synovial macrophages.
Mincle was initially recognized as a downstream target of nuclear factor interleukin-6 (NF-IL-6; also called C/EBPb) in peritoneal macrophages in mice [9]. Human Mincle is expressed by myeloid cells such as circulating monocyte-derived macrophages, macrophages in adipose tissue and BMMCs [6, 10, 11]. Ichioka et al. reported that MINCLE was expressed at higher levels in BMMCs from RA than OA patients [10]. Here, we observed Mincle-positive cells in the synovial lining layer of the knees of KOA patients. CD14+ synovial cells expressed higher levels of MINCLE compared to CD14– cells. A Mincle agonist injection induced skin inflammation in human Mincle Tg mice. Further, a Mincle agonist induced the production of TNF-α in human blood monocyte-derived macrophages, which was completely suppressed by an anti-human Mincle antibody [11]. These findings suggest that Mincle may have a key role in macrophage-related synovial inflammation in KOA.
Mincle is strongly induced by a number of inflammatory stimulation, such as IL-6, lipopolysaccharides (LPS), and interferon (IFN)-γ in macrophages [5, 8, 9]. Treatment with these inflammatory mediators markedly reduced MINCLE mRNA induction in macrophages from NF-IL-6-deficient mice [9]. Additionally, LPS stimulates MINCLE mRNA expression in the murine macrophage cell line RAW264.7 [8]. In the present study, Mincle and TNF-α were localized to the synovial lining layer and stimulation of cultured CD14+ fractions with exogenous TNF-α significantly raised MINCLE mRNA expression compared to vehicle-stimulated cells. Mincle activation leads to the production of chemokines and cytokines, neutrophil recruitment and other inflammatory responses [18]. Mincle signaling promotes inflammation and also maintains the inflammatory state in acute renal inflammation in mice [8]. TNF-α-mediated MINCLE expression may be a key component in synovial inflammation.
In conclusion, Mincle expression was observed in synovial macrophages and was induced by the presence of TNF-α. Mincle may have a key role in maintaining synovial inflammation in the osteoarthritic synovium.


