Purpose
Primary ampullary carcinoma (AC) is a rare malignancy accounting for 0.5-0.6% of all digestive cancers [1, 2]. With the advancements in available imaging technology, their diagnosis (and hence the incidence) has been steadily rising in the last three decades [1]. Although associated with considerable morbidity and mortality, pancreaticoduodenectomy (PD) remains the primary treatment option [3]. Compared with PD, endoscopic papillectomy and transduodenal ampullectomy resulted in fewer complications but a higher recurrence rate, and they apply mainly to ampullary adenoma or some selected early-stage AC patients [4, 5]. However, more than half of cancers of ampulla of Vater were diagnosed at an advanced stage. Therefore, radical resection as a cure was only performed in 40.0-48.3% of cases in the population-based studies [6, 7]. Palliative surgery, which only relieves symptoms without much benefit to the tumor itself, may also cause injury and affect patient’s quality of life. Complicating matters further is that some patients are aged and/or suffer comorbid illnesses, hampering their ability or aspiration to receive timely surgery. Moreover, the effect of adjuvant therapy on ampullary cancer is not well-known [8], and whether to treat it by chemo-radiotherapy is highly controversial. Additionally, there have been very few studies on managing patients with inoperable AC, although they made up more than half of the total [6, 7]. Therefore, unresectable AC patients urgently need effective treatment. Iodine-125 (125I) radioactive seed, which can deliver a high radiation dose to target tissue with reduced damage to surrounding tissues, provides a slow and continuous release of radiation that allows repair of sub-lethal damage and re-oxygenation of hypoxic areas in the late-responding tissues [9]. 125I seed implantation has been reported to be efficient in treating many types of malignant tumors [10, 11], including malignant biliary obstruction in animal and human studies [12-15]. However, the effects of seed implantation in patients with unresectable ampullary carcinoma need further exploration. This study focused on the role of EUS-guided 125I seed implantation in patients with inoperable ampullary carcinoma.
This retrospective study aimed to analyze the feasibility and efficacy of 125I seed implantation performed under EUS in the local control and the long-term outcomes of unresectable ampullary carcinoma patients.
Material and methods
Patients characteristics
From January 2011 to June 2020, 13 ampullary cancer patients (Table 1), including six male and seven female patients, were treated with EUS-guided 125I seed implantation at the Department of Gastroenterology and Hepatology, the First Medical Center of Chinese PLA General Hospital. Mean size of these tumors was 27.46 ± 12.07 mm, at the largest diameter. The inclusion criteria were: 1. Ampullary carcinoma confirmed by pathology; 2. Ineligible for curative surgery or refused surgical resection; and 3. Unwilling to receive external beam radiotherapy (EBRT). Patients with unstable cardio-respiratory function, severe pulmonary or kidney dysfunction, hypo-coagulability, and active infection were excluded from this study. The present research was approved by the Research Ethics Committee of Chinese PLA General Hospital, which complies with the Declaration of Helsinki, and written informed consent was obtained from every patient (approval number: S2021-309-02).
Technical procedures
Treatment protocols
All patients who underwent EUS-guided 125I seed implantation were performed by an experienced endoscopist (Wen Li) with expertise in the technique (Figure 1). Prior to the treatment, all patients underwent a detailed tumor volume evaluation using computed tomography (CT) scans with 1.5 mm thickness within a week before 125I seed implantation. A pre-operative treatment plan was generated for each person based on Paris system implant principles, according to planning target volume to design the number of seeds and the best implantation location.
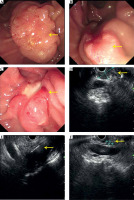
Fig. 1
The procedure of 125I seed implantation. A) The ampullary lesion; B, C) implant bile and pancreatic duct stents; D) The ampullary lesion under EUS; E) Implant seeds under EUS; F) Verifying the place of stent and seeds under X-ray
Since 11 patients suffered clinical or imaging manifestations of bile and/or pancreatic duct obstruction, ERCP was performed in advance, and plastic biliary and/or pancreatic stents were placed (Figure 1A-C). However, two patients suffered duodenal stenosis, and the endoscope could not reach the descending part of the duodenum.
125I seed implantation
The seeds (China Isotope & Radiation Co. Ltd., Beijing, China) were composed of an inactive palladium rod, adsorbed with 125I, and a titanium capsule that enclosed it, producing a 27.4 keV X-ray with a half-life of 59.4 days. Radioactivity per seed of about 0.4 millicuries (mCi) was adopted.
Temperature-sterilized seeds were placed in radiation-resistant metal clips, then installed into a MICK 200-TPV applicator. The applicator was later connected to a 19-gauge fine-needle aspiration needle (COOK, America). EUS (Olympus Corporation, Tokyo, Japan) was used to guide the placement of needles, also including measuring the volume, morphology, and margins of the tumor, along with its’ relationship to surrounding tissues (Figure 1D). Precautions were taken to avoid puncture of the blood vessels and the biliopancreatic tract. The aspiration needle was inserted into the distal edge of the mass under EUS guidance (Figure 1E). The seeds were then implanted in a straight line [16], with a general ensity of 510 seeds/cm, while the needle was drawn backwards. The implant procedure was suspended when the needle was withdrawn to the lateral edge of the lumen 2-3 mm to avoid the seeds escaping into the intestinal lumen during needle extraction. There was no overlap between each needle puncture track (the seed implanted by the previous needle could not be seen in the puncture field of vision). Additionally, the seeds were filling the entire tumor as evenly as possible during implantation. The number of implanted seeds was recorded. Finally, the position and distribution of 125I in lesions were evaluated by fluoroscopy (Figure 1F). A median of 21 seeds (range, 15-40) was implanted per patient, with a median implanted activity of 8.0 mCi (range, 6-16 mCi) in the first treatment. Post-operative adjuvant therapy was generally recommended for all patients, including chemotherapy and molecular-targeted therapy. Regarding radiation safety protection, the safety knowledge was conveyed to patients and their primary family members before and after the treatment.
After the procedure, vital signs were monitored for at least 2 hours, and fasting was maintained for at least 12 hours. Patients were monitored regarding the presence or absence of procedure-related complications.
Radiation protection
All 125I seeds were placed in protective gear before being released, which was performed in a particular area with radiation protection of the endoscopy center. All patients and their primary family members were informed about relative radiation protection before and after the treatment, including lead clothing wearing, safe distance, and monitoring feces (if detached seeds were observed, they were demanded to be collected and sent back by lead box).
Follow-up
Medication was administered when patients complained of pain, abdominal distension, or other symptoms. All patients were hospitalized for at least three days after the procedure, and were monitored by blood routine and liver function at one week, one month, and then at three-month intervals. The patient’s overall condition and appetite were assessed and recorded during the follow-up period. Follow-up imaging (mainly enhanced CT scans) and endoscopy (with EUS, if necessary) were performed every three months. Whether or where to replace additional 125I seeds depended on tumor growth during follow-up visits. If recommended by a medical oncologist and if the patient was willing, post-procedure chemotherapy was allowed in this study during follow-up time. World Health Organization (WHO) criteria were used to assess tumor’s response. Images were evaluated by three endoscopists independently, with findings including complete response (CR), partial response (PR), and stable disease (SD). Disease control rate (DCR) was determined by the sum of CR, PR, and SD rates. Final follow-up visit for this study was conducted in February 2021.
Statistical analysis
All statistical analyses were carried out using SPSS 25 software (IBM, Armonk, NY, USA). Curves were performed by R software (version 4.0.5). Continuous variables were reported as mean and range, or median and interquartile range (IQR). Patients’ cumulative survival time was estimated by Kaplan-Meier curve.
Results
Tumor characteristics and treatment procedures
Tumor diameter ranged from 13.4-50.0 mm, with a mean size of 27.46 mm. In total, 29 sessions of 125I seed implantation were performed in 13 target tumors. Five implantation sessions were done in one tumor, four were performed in three tumors, three in one tumor, two in one tumor, and one implantation session was performed for the remaining seven tumors. The total number of 553 125I seeds was implanted, and the success rate was 100% in 29 sessions of 125I seed implantation.
Adverse events
Severity of the reported toxicity was evaluated using common terminology criteria for adverse events (CTCAE) version 5.0. No procedure-related deaths or fatal complications occurred, and no patients needed to withdraw during these procedures immediately. All of the above-mentioned adverse events were reported as grade 1 and grade 2, and did not require invasive intervention. After 29 sessions of 125I seed implantation, the number of patients experiencing transient abdominal pain, abdominal distension and loss of appetite, and seed migration was five (17.2%), three (10.3%), and one (3.4%), respectively. No post-operative pancreatitis or cholangitis occurred. The procedure-related adverse event rate in this study was 31% (Table 2). The patient, who experienced seed migration in this study was followed-up closely, and no secondary damage was observed.
Table 2
Endoscopic intervention and procedure-related adverse effects
Follow-up and short-time tumor response
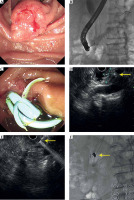
The median follow-up period was 20.5 months, ranging from 3 to 57 months. Six patients received more than two treatments. One patient was lost to follow-up after the procedure, and four patients survived till the last follow-up. One patient experienced tumor progression with a metastatic liver lesion at 9-months follow-up, who accepted chemotherapy. Other patients only received palliative support treatment. CR was observed in one tumor in 6 months, PR was observed in two, seven, seven, and six target tumors in 3, 6, 9, and 12 months respectively, according to EUS. DCR at 3, 6, 9, and 12 months after the first procedure was 100% (12/12), 100% (11/11), 90% (9/10), and 77.8% (7/9) (Table 3). Figure 2 shows tumor changes under endoscopy and EUS before and after the treatment.
Overall survival
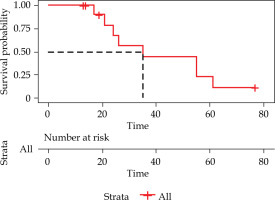
The median OS (from diagnosis to death) time was 35 (95% CI: 8.97-61.03) months (Figure 3). The 1-, 2-, and 5-year OS rates were 100%, 67.5%, and 11.3%, respectively. During a median follow-up of 20.50 (range, 3-57) months, seven patients died of cancer-related cachexia at the end of follow-up, and one died of an unknown cause.
Discussion
Although rare, ampullary carcinoma is the second most common periampullary cancer after pancreatic adenocarcinoma [17]. Although AC is associated with a more favorable prognosis than adenocarcinomas of the adjacent pancreatic duct, common bile duct, or duodenum [18], it is often diagnosed at an advanced stage. Overall, 50.8% of cases were not-resected [6], facing palliative procedure. Radiotherapy, a method to improve local control rate, remains controversial for ampullary carcinoma [19-23]. In fact, most of the conducted studies were based on patients in specialist hospitals. Moreover, almost all of the subjects were patients after radical surgery, which might lead to obvious selection bias. Also, these patients tend to be in their elder age, and PD itself is quite traumatic. Therefore, a combination of radiotherapy and chemotherapy after PD is a great challenge for them, which might affect study results.
One consistency among these retrospective series is another selection bias in recommending an adjuvant treatment in a group. Patients with unfavorable tumors more often received radiotherapy compared with those with more favorable features [24]. Moreover, there is no agreement on the dose of radiotherapy. Studies focusing on radiation therapy in pre-operative or inoperable patients have not been published. Hence, the application of radiotherapy in AC needs further exploring.
The application of conventional external beam radiation therapy (EBRT) may be restricted due to the unique anatomy of Vater, which consists of a terminal portion of the common bile duct and main pancreatic duct. In brachytherapy, 125I seed implantation has significant benefits in destroying hypoxic tumor cells by consistently radiating low-dose rays. Also, it has a significant advantage of delivering high-dose of irradiation to the tumor with a very sharp fall-off outside the implanted volume [9]. 125I seed implantation is used low-dose-rate brachytherapy (LDR-BT), and another brachytherapy is high-dose-rate brachytherapy (HDR-BT) using 192Ir. The evolution of brachytherapy for prostate cancer shows that LDR-BT includes more favorable scheduling planning, lower initial capital equipment costs, non-requirement of a shielded room, completion in a single-implant, and more robust data from clinical trials in comparison with HDR-BT [25]. Outcomes of 125I seed implantation are similar to EBRT, while 125I seed implantation has three advantages [25]: 1. The dose is lower while the irradiation time is longer, effectively destroying tumor cells, and significantly reducing the damage to normal tissues. 2. Continuous irradiation can destroy tumor cells more completely and have a higher biological effect. 3. 125I seed has a short half-life, fewer post-operative complications, and is easy to protect. Also, it has an additional importance that daily visits to the hospital are not needed, which improves patient quality of life [15].
Compared with stent placement alone, 125I seed implantation combined with stent placement [14, 26] or placement of an irradiation stent [27] was reported to be viable treatment that provides survival benefits and prolonged stent patency in patients with malignant biliary obstructions [15]. For patients who are not eligible candidates for conventional radiation, chemotherapy, or surgery, permanent implantation of 125I seeds might be an alternative treatment option.
In this study, we implanted 125I seeds in unresectable AC under EUS. Different from traditional seed implantation for radical treatment, since AC growth can cause obstruction of the pancreas, bile duct, and duodenum, we mainly focused on palliative control of tumor growth, and our follow-up assessment aimed to observe the effect of tumor growth control on the treatment and postponement of obstructions. After treatment, some patients, especially those who had a longer survival time, suffered tumor recurrence, therefore additions of seed implantation were applied to control tumor growth. CR or PR was observed in all patients, except for one who did not reach nine months by the end of follow-up. Among them, eight tumors got complete or partial responses at six months follow-up, and a patient who accepted a second treatment six months later, achieved partial response at 12 months. Time point of local reaction was consistent with iodine seed’s physical characteristic, which includes that 65% of the prescribed dose was delivered in 3 months, and 90% was delivered in about six months [9]. These results indicated that radioactive 125I seeds are beneficial in the local control of ampullary carcinoma. However, two stage IV patients, who once achieved PR died within one year after treatment, and one stage III patient developed liver metastasis nine months after treatment. These also indicated that 125I seed as a local palliative treatment had limitations in the control and prevention of metastasis, especially in advanced patients.
Despite this, our study showed a 5-year OS rate of 11.3%, and was higher than data reported in a population-based study (5-year relative survival rate was 9.5% after palliative surgery and 6.7% after symptomatic treatment) [6]. That possibly suggests there is hope for 125I seed implantation to improve OS in inoperable AC patients. A meta-analysis [28] and our previous study [15] showed that pancreatic cancer with 125I seed implantation brachytherapy had a longer median survival. Since 125I seed implantation is a local treatment, the longer the doubling time, the greater the tumor may benefit. Patients with AC might benefit more in terms of prolonged survival than those with pancreatic cancer. Additionally, the interval between diagnosis and treatment may affect clinical outcomes. In our study, three patients received the seed implant more than 30 months after their initial diagnosis, which might influence the overall survival. The median OS time in our study was 35 (95% CI: 8.97-61.03) months. The mean OS time was 41.4 (95% CI: 28.53-54.29) months. Moreover, the longest survival after the first treatment was 57 months. For the local treatment of inoperable AC, Fowler et al. treated twelve patients with a median survival of 21 months, and the longest survival after the first treatment was only 36 months [29]. Another study that adopted ERCP-guided radio-frequency ablation as the primary therapy reported a mean OS time of 1,081 (95% CI: 757.8-1404.0) days, with four related adverse cases, including mild pancreatitis, bleeding, and late distal biliary stenosis. In comparison with these two studies, this study showed a longer OS time. Nonetheless, it is hard to compare these results directly since there was no tumor stage for all selected patients in their studies, which might affect OS time significantly.
Numerous studies have proven the safety of 125I seed implantation brachytherapy [9, 15, 30]. In this study, 125I seed implantation was performed after ERCP on the same day, so the adverse events mentioned below might contain those caused by ERCP. No procedure-related deaths or fatal adverse events occurred. Adverse events were mild to moderate, and did not require invasive intervention. Transient abdominal pain (5 cases, 17.2%), abdominal distension and loss of appetite (3 cases, 10.3%), and seed migration (1 case, 3.4%) were observed. All procedure-related adverse events were reported in grade 1 and grade 2.
In this study, 125I seed implantation showed good therapeutic effect and safety, and we consider it might be suitable for the following conditions: Unresectable ampullary carcinoma diagnosed by surgical consultation; Patients’ physical condition or age contradict PD, or patients and their families disagree with PD or bypass surgery; No major organ failure, and expected survival of over 3 months. However, since 125I seeds are radioactive and endoscopic implantation is required, some AC patients are not suitable for this treatment. For example, patients and their families do not receive protection training or agree with protection requirements as well as poor general condition that does not allow endoscopic procedures.
This study had some limitations. First, it was a single-center retrospective study, with a small sample size. Second, the optimal dose of irradiation was not determined. Third, due to the complexity of local anatomy, seeds could not be implanted as evenly as other tissue cancers, which is almost impossible to resolve. Fourth, brachytherapy is a local treatment, so we recommend systemic chemotherapy, which may better control tumor growth and prevent metastasis. However, there is no standard chemotherapy plan, and only one case in our study received chemotherapy, so we cannot be sure of its’ influence on the outcome.
In conclusion, our preliminary study suggests EUS-guided 125I seed implantation might control the local tumor progression, and shows the technical feasibility and safety in patients with AC. Nevertheless, further studies are needed to clarify the effectiveness of 125I seed implantation in AC patients.